- 50+ years experience
- Deep subject-matter expertise
- Global Life Sciences network
- Clinical Operations
- Regulatory Affairs
- Medical Affairs
- Biometrics & Data Management
- Drug Safety & Pharmacovigilance
- Quality Assurance
- Supply Chain
- Engineering
- Operations & Maintenance
- Contracting
- Permanent Recruitment
- Interim Solutions
- Blog & News
- Join our Team
- Diversity & Inclusion
- Group Companies
- Life Sciences Investors
- About Executive Search

Home // Clinical Trial Assistant

Clinical Trial Assistant
What does a clinical trial assistant do.
As a Clinical Trial Assistant (CTA) you hold an essential position within a clinical operations team. This might be at a pharmaceutical company, a clinical research organisation or a clinical research unit in a university hospital. The CTA is responsible for supporting clinical drug research and development tasks. You work closely with Clinical Research Associates (CRA), who monitor the progress of the study, and Clinical Project Managers (CPM). You may also work with research nurses, researchers and doctors in hospitals.
You have an important task in study start-up, digital sending, tracing and administration as well as saving essential documents (e.g. case report forms, CVs, study protocols, investigator brochures, informed consent forms and study supplies), and filing important records related to side effects.
Take a look at the Clinical Trial Assistant vacancies to see which are the best fit for you!

Are you looking for a job as Clinical Trial Assistant?
Click here!
What responsibilities can you expect as a Clinical Trial Assistant?
Clinical Trial Assistant roles and responsibilities cover a range of tasks in support of a clinical study. These can include:
- collecting, registering and archiving information and documents in accordance with the applicable good clinical practice guidelines for clinical studies;
- preparing investigator site files, trial master files (TMF) and electronic trial master files (eTMF) for the initiation of participating centres in clinical studies;
- identifying needs, bottlenecks and deviations within your own study team;
- participating in projects or initiatives on request, or on your own initiative;
- taking on department-wide tasks to contribute to the optimisation of processes within the department;
- maintaining the complete documentation for the studies assigned;
- ensuring complete and correct study administration in accordance with the standard operating procedures and the ICH-GCP guidelines;
- submission of clinical trial documents for review and approval by the Ethics Committee.
A CTA job description may describe the position as a ‘Global Clinical Trial Assistant’ or ‘Clinical Trial Assistant Remote’, which will add other roles and responsibilities relevant to the more specialist nature or circumstances of these roles.
Need more details? Browse our Clinical Trial Assistant vacancies

What education and skills do you need as a Clinical Trial Assistant?
As a Clinical Trial Assistant, you hold different responsibilities related to your academic background. Here is a summary of education and skills requirements that a vacancy may mention, as a general guide:
- completed medical, pharmaceutical or administrative education at MBO level, or a higher level (bachelor or master) in Life Sciences;
- excellent computer skills;
- excellent organisational skills;
- knowledge of medical terminology;
- knowledge of GCP;
- good communication skills in Dutch and English.
You may progress to a Senior Clinical Trial Assistant. However, if you want to advance to a CRA, for example, then you need to have a bachelor’s or master’s background.
What salary can you expect as a Clinical Trial Assistant?
Clinical Trial Assistant jobs in the Netherlands usually offer a startsalary ranging from € 30.000,- to € 45.000,- a year depending on your qualifications, skills and experience. There may be additional benefits depending on the contract, and whether you are remote working and/or need to travel.
Do you want to know more about positions as a Clinical Trial Assistant?
View our list of Clinical Trial Assistant vacancies
Is the vacancy you are looking for not listed? Contact us and we will be happy to help you with your dream opportunity! Or see below and sign in.
Sign in with Talentmark
Upload your CV for our recruiters to review.

- On-demand Video Interviews
- Live Interviews and Scheduling
- Applicant Tracking Software
- Interview Questions & Job Descriptions
- Careers Page Builder
- Assessments, evaluation, testing
- AI Assistant & Virtual Recruiter
- Plagiarism Checker
- Integrations
- Mobile Applications
- Collaboration Tools
- Tutorials & Help Center
- Quick Product Tour (2 min video)
- Free Job Description Templates
- ROI Calculator
- Developer Resources
- API Reference
- System Status
- Interview Prep
- Search for jobs
- Resume Builder
- Resume Templates
- Resume Examples
Clinical Trial Assistant Job Description Template
The Clinical Trial Assistant job description template is a document that outlines the essential duties and responsibilities of a clinical trial assistant. This position supports clinical research studies to ensure proper documentation, protocol compliance, and study organization. The template can be used by hiring managers to create a detailed job posting and attract qualified candidates.
Job Summary:
A Clinical Trial Assistant is responsible for providing administrative support to clinical trials in the healthcare industry. They assist medical professionals involved in clinical research by organizing, managing and maintaining clinical trial documentation.
Responsibilities:
- Provide administrative support to clinical trials, including preparation of documents and reports, maintaining trial master files and document storage, and coordinating logistics for meetings, conferences and training sessions.
- Assist with the development of clinical trial protocols, informed consent forms and study reports in accordance with Good Clinical Practice (GCP) and applicable regulations.
- Act as the primary point of contact between study site personnel, sponsors and vendors to ensure timely communication and resolution of issues.
- Track study progress and provide regular updates to study team members and other stakeholders.
- Assist with the preparation and submission of regulatory documents to obtain and maintain trial approval.
- Maintain accurate and up-to-date clinical trial records and ensure compliance with all relevant regulations and guidelines, including FDA, ICH and GCP.
- Participate in study team meetings and provide input on trial design and execution.
Requirements:
- Bachelor's degree in a scientific or healthcare related field, or equivalent experience.
- At least 1-2 years of experience supporting clinical trials in a healthcare or pharmaceutical setting.
- Knowledge of GCP, FDA regulations, and other relevant guidelines and regulations governing clinical trials.
- Excellent organizational, communication and interpersonal skills.
- Ability to work effectively in a team-oriented environment with multiple stakeholders.
- Proficient in Microsoft Office Suite and other database software.
- Experience with electronic data capture systems is preferred.
Introduction
A clinical trial assistant (CTA) plays a vital role in supporting clinical research teams in conducting trials. Therefore, hiring the right CTA is imperative to ensure the efficient functioning of clinical trials. This article provides you with a step-by-step guide on how to create a CTA job posting that will attract the right candidates.
Job Title and Job Summary
Your job posting should have a clear title that accurately summarizes the job role. Your CTA job title should be "Clinical Trial Assistant." In the job summary, you should describe the main responsibilities of the job. For instance:
- Assisting in study start-up activities, site monitoring, and study close-out.
- Preparing regulatory documents and ensuring compliance with regulatory guidelines.
- Performing administrative tasks such as filing, data entry, and coordinating meetings.
- Assisting in participant recruitment and screening activities.
- Managing study supplies and ensuring timely deliveries to study sites.
Qualifications and Experience
In this section, you should outline the required qualifications and experience that an ideal candidate should have. For instance:
- Bachelor’s degree in a relevant field.
- Experience working in a clinical research environment.
- Knowledge of regulatory guidelines such as ICH-GCP and FDA regulations.
- Excellent organizational and communication skills.
- Strong attention to detail and ability to work under pressure.
Working Conditions
Here, you should provide information about the working conditions that the candidate will be working in. For instance:
- The job is primarily office-based, but some travel may be required.
- May be required to work overtime to meet deadlines.
- The candidate should be willing to work as part of a team and also independently.
Benefits and Salary
This section should give a brief overview of the benefits that the candidate will receive and the salary range for the job. For instance:
- The successful candidate will be offered a competitive salary.
- Other benefits include health insurance, pension, and generous vacation time.
In conclusion, creating a well-written job posting for a CTA position will attract the right candidates and help you find the perfect fit for your clinical research team. Remember to be clear and concise in your job posting and highlight the key requirements of the job.
Hiring a Clinical Trial Assistant: Frequently Asked Questions
What is a clinical trial assistant.
A Clinical Trial Assistant is responsible for providing administrative support to clinical research teams. They perform various tasks such as maintaining study files, tracking study progress, and preparing trial documents. A Clinical Trial Assistant is expected to be detail-oriented, organized, and possess excellent communication and problem-solving skills.
What qualifications should a Clinical Trial Assistant have?
A Clinical Trial Assistant should hold a Bachelor's degree, preferably in life sciences, nursing, or a related field. Additionally, experience in clinical research, project management, or administrative support is often required. Along with education and experience, important qualities for a Clinical Trial Assistant include strong attention to detail, excellent communication skills, and the ability to manage time effectively.
What are the responsibilities of a Clinical Trial Assistant?
- Maintaining and organizing study files and documents
- Tracking study progress and ensuring that study milestones are met within deadlines
- Coordinating and scheduling study-related activities, such as investigator meetings and site visits
- Assisting in the preparation of study-related reports, presentations, and documentation
- Communicating with investigators and other team members to ensure that study requirements are being met
What are some key skills required for a Clinical Trial Assistant?
A Clinical Trial Assistant should possess excellent organizational and problem-solving skills. They must be able to work independently and manage multiple tasks within tight deadlines. Strong proficiency in Microsoft Office is often required. Furthermore, effective communication skills are a must-have, as a Clinical Trial Assistant must be able to work and communicate effectively with all team members.
What is the typical salary for a Clinical Trial Assistant?
Salaries for Clinical Trial Assistants can vary depending on the level of experience, location, and company size. According to Glassdoor, the average salary for a Clinical Trial Assistant in the United States is $52,274 per year.
What are some tips for creating a Clinical Trial Assistant job posting?
- Use clear and concise language and avoid buzzwords or jargon
- Clearly state the job requirements and qualifications
- Provide a detailed description of the responsibilities and duties of the role
- Include information about the company culture and values to attract the right candidates
- Be transparent about the salary range and benefits offered
Related Job Descriptions
Start saving time and money on recruiting.
Start today for free to discover how we can help you hire the best talents.

This site uses cookies to make it work properly, help us to understand how it’s used and to display content that is more relevant to you. For more information, see our Privacy Policy
Protect your data
This site uses cookies and related technologies for site operation, and analytics as described in our Privacy Policy . You may choose to consent to our use of these technologies, reject non-essential technologies, or further manage your preferences.
- Resume and Cover Letter
- Research Assistant Job...
Research Assistant Job Description: All Key Roles & Duties
6 min read · Updated on June 03, 2024

When you want to land a Research Assistant job, the job description is your best friend.
In order to ensure your professional resume will support your goals, use this Research Assistant job description to inform what you should highlight on your resume.
By reviewing job description roles and duties, you'll be able to identify what technical and soft skills , credentials, and work experience matter most to an employer in your target field.
Research Assistant Job Description
Participate in the design, administration, and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives.
May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related area. Knowledge of FDA regulatory requirements is required. Has knowledge of commonly-used concepts, practices, and procedures within a particular field. Rely on instructions and pre-established guidelines to perform the functions of the job. Work under immediate supervision. Primary job functions do not typically require exercising independent judgment. Typically reports to a supervisor or manager.
Responsibilities:
Conduct literature reviews
Collect and analyze data
Prepare materials for submission to granting agencies and foundations
Prepare interview questions
Recruit and/or interview subjects
Maintain accurate records of interviews, safeguarding the confidentiality of subjects as necessary
Summarize interviews
Provide ready access to all experimental data for the faculty researcher and/or supervisor
Request or acquire equipment or supplies necessary for the project
Manage and respond to project-related email
Prepare, maintain, and update website materials
Supervise undergraduate students working on the research project (maintaining records on assignment completion, acting as liaison/mediator between the undergraduate students and the faculty researcher)
Attend project meetings
Attend area seminars and other meetings as necessary
Summarize project results
Prepare progress reports
Prepare other articles, reports, and presentations
Monitor the project budget
Travel to field sites to collect and record data and/or samples as appropriate to the specific objectives of the study
As appropriate to the specified position, code and verify data in accordance with specified research protocol and coding procedures and enter data into a computer database and/or spreadsheet application for subsequent analysis
Develop or assist in the development of interview schedules; contact potential subjects to introduce and explain study objectives and protocol and to arrange interviews, either in person or by telephone
Identify and compile lists of potential research subjects in accordance with study objectives and parameters, as appropriate to the individual position
Conduct and record face-to-face and/or telephone interviews with subjects, in accordance with predetermined interview protocol, data collection procedures and documentation standards
Review and edit data to ensure completeness and accuracy of information; follow up with subjects to resolve problems or clarify data collected
May set up, calibrate and maintain laboratory and/or field research equipment, as specified by the requirements of the study
May lead or guide the work of student employees
Perform miscellaneous job-related duties as assigned
Prepare findings for publication and assist in laboratory analysis, quality control, or data management
Write and contribute to publications
Develop research protocols
Track progress over time
Assist with preparation of all educational and training workshops and evaluation strategies
Engage clinical and community partners in research
Market training and technical assistance resources to clinical partners and academic investigators
Develop assessment and evaluation tools
Compile data for progress reports
Requirements:
Completed degree(s) from an accredited institution that are above the minimum education requirement may be substituted for experience on a year for year basis
High school diploma or equivalent; college degree preferred
Tailor your resume
As you read through the Research Assistant job description, you likely noticed there are things the employer wants that you absolutely know how to do. You should make a list of the skills you have so they align with the duties you'll need to perform in your new job.
As an example, the first responsibility listed in this Research Assistant job description is the ability to “conduct literature reviews.” You will want that exact verbiage in your resume. Tailoring your Research Assistant resume with the language you find in the job description will help your resume get past the applicant tracking system.
The applicant tracking system – ATS
Make no mistake: almost every job you apply to will push your resume through the ATS before it gets into the hands of a hiring manager. It will scan your resume for everything from experience level and education to keywords.
Let's take a look at some of the skills you should consider including in your Research Assistant resume.
Research Assistant top skills & proficiencies
By using the exact wording from the Research Assistant job description, you'll be speaking directly to the bot through relevant keywords. Use those skill-related keywords and make sure you have a good balance of hard and soft skills .
Hard skills are things you know how to do because of education, on-the-job training, and experience. In other words, they're what you've learned over the course of your career. It's pretty easy to distinguish them because they are quantifiable. Meaning you can put a number with them.
For example, if you're using “data collection” as one of your hard skills, you can quantify it by saying that you “collect data from 3 disparate sources or data lakes to compile actionable reports for senior leaders.”
Soft skills are not often quantifiable. These types of skills are the interpersonal abilities you possess that allow you to get along with others and solve problems.
Communication
Attention to detail
Critical thinking
Planning and scheduling
Interviewing
Data collection
Conflict resolution
Related reading: 47 Accomplishment Examples for Your Resume: Expert Picks
The Research Assistant job description is the key to job search success
At the end of the day, the goal of applying for any job is to win an interview. By using the Research Assistant job description, you'll get past the ATS and impress the hiring manager.
If you want to be certain that you've got the right skills and keywords in your resume from the Research Assistant job description, TopResume will perform a free resume review for you.
Recommended reading
5 Simple Steps to Customizing Your Resume for Each Job
How to Write a Targeted Resume That Lands You an Interview
How to Customize Your Job Application for a Specific Job Listing
Related Articles:
7 Signs Your Resume is Making You Look Old
Why a Simple Resume Layout is a Successful Resume
Software Developer Top Needed Skills
See how your resume stacks up.
Career Advice Newsletter
Our experts gather the best career & resume tips weekly. Delivered weekly, always free.
Thanks! Career advice is on its way.
Share this article:
Let's stay in touch.
Subscribe today to get job tips and career advice that will come in handy.
Your information is secure. Please read our privacy policy for more information.

Clinical Trials Assistant Job Description [Updated for 2024]
In the healthcare sector, the demand for Clinical Trials Assistants is continually growing.
As medical advancements progress, the need for skilled individuals who can organize, coordinate, and supervise clinical trials becomes more pressing.
But let’s delve deeper: What’s really expected from a Clinical Trials Assistant?
Whether you are:
- A job seeker trying to understand the core of this role,
- A hiring manager outlining the perfect candidate,
- Or simply fascinated by the complexities of clinical trials,
You’ve come to the right place.
Today, we introduce a customizable Clinical Trials Assistant job description template, designed for convenient posting on job boards or career sites.
Let’s dive right into it.
Clinical Trials Assistant Duties and Responsibilities
Clinical Trials Assistants aid in conducting clinical trials, which are part of the process to test new drugs and medical devices for efficacy and safety.
They are responsible for administrative and scientific tasks which contribute to the overall trial.
The duties and responsibilities of a Clinical Trials Assistant include:
- Assisting in the coordination and administration of clinical trials research studies
- Collecting, transcribing and maintaining clinical trial data and patient information
- Preparing and maintaining documentation and reports for clinical trials
- Assisting with the development of research study budgets
- Organizing, managing and filing clinical trial documentation and reports
- Ensuring compliance with standard operating procedures, FDA regulations and international standards for clinical trial conduct
- Coordinating and scheduling trial participants’ appointments
- Communicating with trial participants and addressing their questions or concerns
- Assisting in the preparation and submission of protocol consent forms, amendments, and serious adverse event reports to ethics committees
- Supporting the implementation of trial protocols, working closely with other clinical staff
Clinical Trials Assistant Job Description Template
We are seeking a detail-oriented Clinical Trials Assistant to join our team.
Your role will involve supporting the Clinical Trials Manager in the execution and coordination of clinical trials, ensuring all projects adhere to regulatory and ethical standards.
Our ideal candidate will have previous experience in clinical research, excellent data management skills, and a strong understanding of Good Clinical Practice (GCP) guidelines.
Ultimately, the Clinical Trials Assistant’s role is to ensure the smooth and compliant running of our clinical trials, contributing to the development of safe and effective healthcare solutions.
Responsibilities
- Assist in the coordination and administration of clinical trials
- Maintain comprehensive records of all trial documentation
- Prepare and organize patient recruitment materials
- Assist in the development and implementation of patient recruitment strategies
- Monitor trial progress and ensure compliance with protocol
- Collect, process and store patient data in line with GCP
- Coordinate logistics for trial materials and equipment
- Liaise with trial stakeholders including investigators, patients, and sponsors
- Prepare trial status reports and keep all stakeholders informed
Qualifications
- Previous experience in a clinical research setting
- Familiarity with GCP and ethical guidelines for clinical research
- Excellent organizational and communication skills
- Ability to handle sensitive and confidential information
- Strong data management skills
- Attention to detail and ability to multitask
- Bachelor’s degree in Life Sciences, Nursing, or related field
- Health insurance
- Dental insurance
- Retirement plan
- Paid time off
- Professional development opportunities
Additional Information
- Job Title: Clinical Trials Assistant
- Work Environment: Office setting within a healthcare facility. Some travel may be required for site visits or conferences.
- Reporting Structure: Reports to the Clinical Trials Manager or Director of Clinical Research.
- Salary: Salary is based upon candidate experience and qualifications, as well as market and business considerations.
- Pay Range: $45,000 minimum to $65,000 maximum
- Location: [City, State] (specify the location or indicate if remote)
- Employment Type: Full-time
- Equal Opportunity Statement: We are an equal opportunity employer and value diversity at our company. We do not discriminate on the basis of race, religion, color, national origin, gender, sexual orientation, age, marital status, veteran status, or disability status.
- Application Instructions: Please submit your resume and a cover letter outlining your qualifications and experience to [email address or application portal].
What Does a Clinical Trials Assistant Do?
Clinical Trials Assistants typically work in the medical and pharmaceutical industries.
They are an integral part of research teams conducting scientific studies to test the efficacy and safety of new drugs or medical treatments.
They are responsible for providing administrative support to Clinical Research Coordinators and Investigators, which may involve tasks like scheduling appointments, maintaining patient records, and managing databases related to the trials.
Clinical Trials Assistants collect and process data, ensure accuracy, and prepare reports based on the results.
They may also assist with participant recruitment and consent procedures, ensuring that all participants are fully informed of the trial process and their rights.
They play a critical role in the coordination and execution of clinical trials, ensuring compliance with study protocols, ethical guidelines, and regulatory requirements.
Moreover, they may also assist in the preparation and handling of trial supplies and materials, and in the management of trial data and results.
Clinical Trials Assistants also liaise with healthcare professionals, patients, and other stakeholders, assisting with site management and monitoring activities.
Their role is crucial in facilitating the smooth running of clinical trials, ensuring the validity and integrity of the data collected.
Clinical Trials Assistant Qualifications and Skills
A proficient Clinical Trials Assistant should possess the following skills and qualifications to ensure efficient and effective performance in their role:
- Excellent knowledge of Good Clinical Practice (GCP) guidelines and the ability to adhere to them to ensure the ethical and scientific integrity of clinical trials.
- Strong organizational skills to manage multiple clinical trials and maintain accurate records related to the trials.
- Ability to work in a team and effectively communicate with other healthcare professionals, patients, and their family members.
- Strong attention to detail to ensure all trial procedures are followed as per the trial protocol and all data is accurately collected and reported.
- Ability to handle sensitive information, respecting patient confidentiality at all times.
- Competent in using various data management systems and software for efficient and accurate record keeping and reporting.
- Good interpersonal skills to build strong relationships with trial participants, ensuring their comfort and understanding of the trial process.
- Problem-solving skills to identify and respond to any issues or irregularities that arise during the trial process.
Clinical Trials Assistant Experience Requirements
Clinical Trials Assistants typically need to have a minimum of 1 to 2 years of experience in a healthcare or clinical research environment.
This experience could be gained through internships, part-time roles, or even full-time work in a related field such as nursing, medical technology, or laboratory science.
Entry-level Clinical Trials Assistants might also have experience from clinical research courses, where they have learned the fundamentals of clinical trial processes, ethical considerations, data management, and regulatory compliance.
Candidates with more than 3 years of experience may have been involved in a range of clinical trials, gained deeper knowledge of trial protocol development and implementation, and have had direct involvement in patient data collection and management.
Clinical Trials Assistants with more than 5 years of experience are likely to have mastered the technical aspects of clinical trials and may have begun to take on leadership roles.
Such candidates could be ready for a managerial position, overseeing a team of assistants and coordinating multiple trials simultaneously.
In addition to practical experience, many roles require a Clinical Trials Assistant to have relevant certifications, including Good Clinical Practice (GCP) and training in the protection of human research participants.
Clinical Trials Assistant Education and Training Requirements
Clinical Trials Assistants typically need a bachelor’s degree in life sciences, nursing, or a related field.
They are required to have a strong understanding of clinical research protocols, regulatory requirements, and the overall clinical trial process.
Knowledge about good clinical practices (GCP) and ethical guidelines is also essential.
Some positions may demand prior experience in clinical research coordination or a similar role, often requiring hands-on experience in data collection, management, and report generation in a clinical environment.
Certification as a Clinical Research Associate (CCRA) or a Clinical Research Coordinator (CCRC) can be beneficial for career advancement and are sometimes preferred by employers.
These certifications can be obtained through organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SoCRA).
Additional training in medical terminology, patient recruitment, data collection and data management tools, and ethics in clinical research may also be required.
Master’s degree in Clinical Research, Public Health or other related fields can add value and may be preferred for senior-level positions.
Ongoing training and professional development are crucial in this field due to the continuous evolution of clinical research regulations and best practices.
Clinical Trials Assistant Salary Expectations
A Clinical Trials Assistant earns an average salary of $47,861 (USD) per year.
However, this can fluctuate based on factors such as previous experience in the field, the region in which they are working, and the specific company that employs them.
Clinical Trials Assistant Job Description FAQs
What skills does a clinical trials assistant need.
A Clinical Trials Assistant needs strong organizational skills to manage multiple tasks and maintain detailed records of trial data.
They also need good interpersonal skills to liaise between patients, researchers, and medical professionals.
A working knowledge of Good Clinical Practice (GCP) guidelines is a must, as well as the ability to work under pressure and meet tight deadlines.
Do Clinical Trials Assistants need a degree?
Most positions require a Bachelor’s degree in a life science or a related field.
Relevant work experience, such as experience in clinical research or healthcare, may be accepted in place of educational requirements for some positions.
Some roles may also require a Clinical Research Coordinator (CRC) certification.
What should you look for in a Clinical Trials Assistant resume?
Look for a strong educational background in a related life science field and any relevant work experience, particularly in clinical research or healthcare.
Other desirable features can include experience with data entry or management, familiarity with GCP guidelines, and any certifications related to clinical research.
Check if the candidate has experience working with a diverse patient population and a multidisciplinary team.
What qualities make a good Clinical Trials Assistant?
A good Clinical Trials Assistant is highly organized, as they will need to manage and track extensive amounts of data.
They need to be meticulous and detail-oriented to ensure the accuracy of this data.
It’s also important that they have strong communication skills, as they will need to liaise between various stakeholders including researchers, patients, and medical professionals.
Is it difficult to hire a Clinical Trials Assistant?
Hiring a Clinical Trials Assistant can be challenging due to the specialized nature of the role.
The candidate must have a certain level of educational background, relevant work experience, and a good understanding of clinical research protocols.
It can be difficult to find individuals with the right mix of skills and experience.
Offering competitive salaries and opportunities for professional development can help attract qualified candidates.
And there we have it.
Today, we’ve delved into the intricate world of a clinical trials assistant.
Guess what?
It’s not just about paperwork.
It’s about participating in the development of life-changing medical treatments and contributing to the advancement of healthcare.
With our comprehensive clinical trials assistant job description template and real-life examples, you’re fully equipped to take the next step.
But why stop here?
Go further with our job description generator . It’s your ultimate tool for creating detailed job listings or enhancing your resume to perfection.
Every task you undertake as a clinical trials assistant plays a crucial role in the grand scheme of medical advancements.
Let’s revolutionize healthcare. Together.
How to Become a Clinical Trials Assistant (Complete Guide)
Skip the Stress: Surprisingly Simple Jobs with Surprisingly Big Payoffs!
Happiness at Work: The Most Satisfying Jobs to Pursue
The Unusual Workday: Jobs That Break Every Stereotype
Fun and Fortune: Enjoyable Jobs That Also Pay Well
The Editorial Team at InterviewGuy.com is composed of certified interview coaches, seasoned HR professionals, and industry insiders. With decades of collective expertise and access to an unparalleled database of interview questions, we are dedicated to empowering job seekers. Our content meets real-time industry demands, ensuring readers receive timely, accurate, and actionable advice. We value our readers' insights and encourage feedback, corrections, and questions to maintain the highest level of accuracy and relevance.
Similar Posts
![clinical trial research assistant job description Android App Developer Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/04/android-app-developer-job-description-768x512.webp)
Android App Developer Job Description [Updated for 2024]
![clinical trial research assistant job description Glass Equipment Supplier Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/07/glass-equipment-supplier-job-description-768x512.webp)
Glass Equipment Supplier Job Description [Updated for 2024]
![clinical trial research assistant job description Customs Brokerage Clerk Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/05/customs-brokerage-clerk-job-description-768x512.webp)
Customs Brokerage Clerk Job Description [Updated for 2024]
![clinical trial research assistant job description Truck Driver Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/02/truck-driver-job-description-768x512.webp)
Truck Driver Job Description [Updated for 2024]
![clinical trial research assistant job description Craft Supply Store Manager Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/05/craft-supply-store-manager-job-description-768x512.webp)
Craft Supply Store Manager Job Description [Updated for 2024]
![clinical trial research assistant job description Foreign Language Department Head Job Description [Updated for 2024]](https://interviewguy.com/wp-content/uploads/2024/07/foreign-language-department-head-job-description-768x512.webp)
Foreign Language Department Head Job Description [Updated for 2024]
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

Clinical Trial Assistant Job Description
- Maintenance Jobs
- ')" data-event="social share" data-info="Pinterest" aria-label="Share on Pinterest">
- ')" data-event="social share" data-info="Reddit" aria-label="Share on Reddit">
- ')" data-event="social share" data-info="Flipboard" aria-label="Share on Flipboard">
What Is a Medical Office Assistant?
Minimum education requirements for a clinical research coordinator, what are the duties of a medical assistant in an office or hospital setting.
- Medical Auditor Jobs
- How Much Do Medical Malpractice Lawyers Make?
Clinical trials are designed to test new medications and medical devices for safety and efficacy. Trials are highly regulated and thoroughly monitored, which results in a significant record-keeping and compliance burden. Many organizations running trials hire clinical trial assistants as support staff. In most cases, clinical trial assistants work in administrative positions. Depending on the setting and the trial, some CTAs might have also basic medical duties such as taking blood or performing lab tests.
Clinical Trial Assistant Responsibilities
Clinical research assistant duties might include archiving and organizing clinical trial master file documents, including clinical study site documents, email correspondence, training materials and other study documents. They also generate filing indexes and maintain currents lists of correspondence. According to Glassdoor , CTA job description duties typically include the expectation that these professionals should administer clinical trials according to good clinical practices and standard operating procedures, and work as a liaison with review boards and independent ethics committees. They might act as key contacts between the contract laboratory, study team and study site. CTAs also provide general logistical support for clinical trials, such as creating an agenda and taking minutes at meetings, and assisting with purchasing and/or budget activities.
Clinical Trial Assistant Education
According to CCRPS , the typical education requirement for a clinical trial assistant is at least an associate degree in a health sciences field, though a growing number of employers require a bachelor's degree. CTAs typically have academic backgrounds in areas such as nursing, pharmacy, health care management or life sciences research, so consider majors related to these areas of study. Lab classes and statistics classes are also important for CTAs. Look for opportunities to work in chemistry or biology labs, and maybe even consider some campus work study in the lab so that you graduate with the experience necessary to land a job.
Administrative and Computer Skills
CTAs should be highly proficient in software applications such as Word, Excel and PowerPoint. Good written and verbal communication skills are also required to work as a CTA, as is an ability to multitask. Experience with health insurance and/or medical office management is considered a big plus.
Basic Database Skills
Clinical trials are all about data, and CTAs are expected to have strong database skills. They must maintain and update site demographics on computer databases. They must also retrieve data and answer questions pertaining to certain databases. CTAs might be tasked with logging in received clinical research forms and transferring data from the forms into the database. In some cases, senior CTAs are put in charge of trial document control, which includes maintaining master files and version control.
- CCRPS: How To Become a Clinical Research Assistant
- Glassdoor: Clinical Research Associate Job Description
Related Articles
Clinical data entry associate job description, responsibilities of a clinical director in assisted living, medical laboratory assistant job description, what are the duties of a litigation paralegal, what experience do you need to be an archaeologist, clinical director's job description & skills list, the requirements for clia laboratory surveyor, what duties can be delegated to a paralegal, the role of a deputy prosecuting attorney, most popular.
- 1 Clinical Data Entry Associate Job Description
- 2 Responsibilities of a Clinical Director in Assisted Living
- 3 Medical Laboratory Assistant Job Description
- 4 What Are the Duties of a Litigation Paralegal?

Clinical Research Assistant Job Description, Key Duties and Responsibilities
This post presents detailed information on the clinical research assistant job description, including the key duties, tasks, and responsibilities they commonly perform.
It also highlights the important requirements you may be asked to fulfill to be hired for the clinical research assistant role by most recruiter/employer.
What Does a Clinical Research Assistant Do?
A clinical research assistant, CRA, is responsible for providing quality patient care and service to the clinical research clients and the healthcare providers.
The clinical research assistant job description involves working closely with doctors, nurses, and other technicians in performing clinical and laboratory procedures.
It also entails conducting pre-study testing in accordance with protocols.
Clinical research assistants also document results of testing in a medical record of the assigned patient and notify medical staff, X-ray technicians, lab personnel, or nursing staff immediately if there is any unexpected result discovered during their daily duties.
They provide technical expertise by maintaining a professional attitude towards clients and all healthcare providers within their scope of practice in accordance to guidelines, regulations, programs of care (POC) and established business practices.
It is also their responsibility to provide regular reports on patients’ condition, including vital signs and symptoms.
They collect and record data in compliance with regulatory requirements and ensure that data is stored and maintained in a secure location at the site of care.
Clinical research assistants create records by documenting their various activities throughout the day, which requires medical terminology to describe findings or procedures performed during the day on patients of all ages or illnesses.
They maintain records of patient care, procedures, and treatments to ensure accuracy, as well as provide a record for future reference.
CRAs ensure that all patient records are maintained in a secure location and in accordance to HIPAA policies and guidelines.
One of their duties involve assisting patients by providing or arranging transportation, such as ambulances or wheelchairs; helping them with eating or drinking; providing comfort measures, medication reminders and information on medical insurance coverage.
Clinical research assistants work closely with staff as they develop and implement a plan to meet the study’s objectives and goals.
Providing a variety of services to staff and ancillary departments, such as food service, laundry, housekeeping and security departments is also part of their duties.
The clinical research assistant work description may entail maintaining records of findings or procedures performed during studies at all times.
It also involves maintaining regulatory compliance by following government regulations, research protocols, and business practices to ensure that the organization is operating in accordance with current industry standards.
Clinical research assistants work in accordance with the policies of the facility where they are employed and will ensure that those policies are adhered to at all times.
They assist clinical laboratory staff in performing tests and procedures, such as taking blood, drawing blood, processing blood, and providing other lab services.
Attending scheduled training seminars, workshops, and other educational courses is a part of their tasks.
Clinical research assistants must maintain a professional attitude at all times and conduct themselves in a manner that is consistent with the standards of the medical industry.
They provide high quality patient care and service to the clinical research clients and healthcare providers.
It is their duty to ensure that all patient records are maintained to ensure accuracy and provide a record for future reference.
They work closely with the physicians, nurses, other technicians and patients in performing clinical and laboratory procedures.
Clinical Research Assistant Job Description Sample/Example/Template
The clinical research assistant job description consists of the following duties, tasks, and responsibilities:
- Conducts patient evaluation
- Organizes patient visits
- Provides patient care in a variety of settings (e.g., physician office, university clinic, hospital)
- Performs demographic and physical assessments of patients
- Provides patient education
- Collaborates with other clinical research assistants and staff within the department
- Collects data for study protocols
- Maintains medical records
- Conducts quality review of medical records and research data
- Provides study-related information to patients during research studies
- Prepares patients for procedures/treatments
- Prepares study-related materials using laboratory equipment (e.g., blood pressure cuff, stethoscope, and otoscope)
- Takes blood samples from patients for testing (e.g., EKG, x-rays, laboratory tests)
- Collects specimens (e.g., urine samples) from patients for testing (e.g., urine test)
- Supports other clinical research assistants
- Documents observations and information generated during patient care
- Performs clerical tasks (e.g., data entry, typing, etc.)
- Continues health education with patients
- Assists nurses in the clinic setting or on hospital units as needed for a variety of tasks (e.g., patient care, record keeping).
Clinical Research Assistant Job Description for Resume
If you have worked before as a clinical research assistant or are presently working in that role and are making a resume or CV for a new job, you can make the Professional Experience section for the resume by applying the sample clinical research assistant job description provided above.
You can express the duties and responsibilities you have performed as a clinical research assistant or are presently carrying out by applying the ones provided in the above clinical research assistant job description template.
This can easily convince the employer/recruiter that you have been effective on the clinical research assistant job, which can greatly influence them to grant you an interview and hire you for the job that you are seeking, especially if it requires having some clinical research assistant work experience.
Clinical Research Assistant Requirements: Skills, Knowledge, and Abilities for Career Success
Here are important requirements you may be expected to meet to be hired by most employers/recruiters for the clinical research assistant role:
- Good oral and written communication skills
- Good time management and organizational skills
- Supervisory capacity and ability to take direction from superiors
- Computer literacy in working with Excel and Word
- Basic mathematics, typing, and computer knowledge
- Ability to pay attention to details in work environment (e.g., accuracy of data collection)
- Travel required throughout the country
- Bachelor’s degree in medical field (e.g., medicine, nursing, public health)
- License to practice in the state of employment (e.g., state of Florida)
- Ability to communicate and relate effectively with co-workers and patients
- Valid driver’s license
- Excellent customer service skills
- Ability to work full time, holidays, and overtime when needed.
Clinical Research Assistants Salary
According to Glassdoor, the national average compensation for a clinical research assistant in the United States is $41,677 per year.
The role of the CRA offers a person with an interest in scientific research and patient care a unique opportunity for employment.
Clinical research assistants are an integral part of the process that moves medical discoveries from research to application with patients.
The clinical research assistant also provides highly skilled professional service to clients and healthcare providers.
This post is useful to individuals who are interested in the clinical research assistant career.
They will be able to learn all they need to know about the duties and responsibilities of the job and so be able to decide if it’s the right career for them.
It is also helpful to recruiters/employers in making a detailed job description for the clinical research assistant position in their organizations for use in hiring for the role.
Recommended:

This Site Uses Cookies
Privacy overview.
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
Clinical Trials Assistant Job Description
Clinical trials assistant duties & responsibilities.
To write an effective clinical trials assistant job description, begin by listing detailed duties, responsibilities and expectations. We have included clinical trials assistant job description templates that you can modify and use.
Sample responsibilities for this position include:
Clinical Trials Assistant Qualifications
Qualifications for a job description may include education, certification, and experience.
Licensing or Certifications for Clinical Trials Assistant
List any licenses or certifications required by the position: NCCPA, ACLS, BLS, MA, CCRC, CA, IV
Education for Clinical Trials Assistant
Typically a job would require a certain level of education.
Employers hiring for the clinical trials assistant job most commonly would prefer for their future employee to have a relevant degree such as Bachelor's and Associate Degree in Business/Administration, Biostatistics, Management, Health Care Administration, Education, Nursing, Healthcare, Science, Pharmacy, Public Health
Skills for Clinical Trials Assistant
Desired skills for clinical trials assistant include:
Desired experience for clinical trials assistant includes:
Clinical Trials Assistant Examples
- Microsoft Word (.docx) .DOCX
- PDF Document (.pdf) .PDF
- Image File (.png) .PNG
- Contribute to, and participate in, study dry runs, pre-study planning activities, departmental and project specific meetings
- Prepare and send CDAs, and other study related materials to clinical study sites
- Coordinate in-house blood draws and internal sample collection studies
- Participate in the development andrevision of documents and study support materials
- Track progress of projects and identify appropriate actions to achievetarget objectives
- Reviewdocuments for completeness and accuracy
- Maintain relationship with sponsors andstudy sites throughout trials
- Implement the standards for research protocols in compliance with regulatory, institutional and external agencies
- Clinical responsibilities include monitoring toxicities, assessing clinical responses and providing exemplary patient care while ensuring the protocol is conducted in a high quality manner
- Offer patient education and patient/family support
- High or Secondary School diploma/certificate or country’s educational equivalent and 3 years administrative support experience
- Good written and verbal communication skills including good command of German and English language
- Bachelor’s degree in life science or related field and 3-4 years clinical research experience, or equivalent combination of education, training and experience
- Bachelor/Master in life science related study program or country’s educational equivalent and administrative support experience
- School diploma/certificate or educational equivalent
- Minimum 1 year of clinical monitoring experience in the pharmaceutical / CRO industry
- Collaborate with Clinical Project Manager (CPM), CRS/iCRS, CRAs/iCRAs and RSU on the preparation, handling, distribution, filing, and archiving of clinical documentation and reports according to the scope of work and standard operating procedures
- May perform assigned administrative duties to support team members with clinical trial execution
- Collaborate with Clinical Project Manager (CPM), CRAs/iCRAs and RSU on the preparation, handling, distribution, filing, and archiving of clinical documentation and reports according to the scope of work and standard operating procedures
- Assist with periodic review of study files and completeness
- Hire, supervise, coach, mentor, and manage performance of the supervisory staff in the office and provide oversight of the staff who report to them
- Establish and maintain an organizational structure that optimally supports the conduct and support of clinical trials in the Center
- Support the UACC Director and Associate Director of Administration in driving unifying clinical development strategies across the two campuses, Tucson and Phoenix
- Support the Deputy Director of the UACC in the running of the UACC-Phoenix, including hiring, grants, space, and clinical trials
- Develop and maintain Clinical Trials Office policies, procedures, work instructions, and other resource information that allows the staff to perform efficiently and to deliver a high quality work product
- Oversee multiple areas within the UACC-Phoenix Clinical Trials Office, accountable for ensuring excellence and compliance in areas of human resources, clinical trials, grants/contracts management, public relations/communications, philanthropy, and facility management
- You may accompany Clinical Research Associates on site visits to assist with clinical monitoring duties upon completion of required training and with required approval
- Please only add information if it is applicable to the vacant job
- Clinical research nursing experience preferred
- Bachelor’s degree or equivalent in the life sciences or related field required
- Related research experience strongly preferred
- Clinical documents
- Organize academic conferences that focus on disease or research dependent topics
- Work closely with the UACC-Tucson Operations to synergize clinical trials efforts across the two UACC campuses
- Develop, implement, and maintain effective staff training programs
- Develop and implement metrics for productivity and quality
- Provide input to the development of annual budgets for the clinical trials operation, monitor financial status, and take appropriate actions required to manage to budget
- Serve as a liaison to college and university offices, state and federal agencies, affiliated programs and industry sponsors related to the conduct of clinical research
- Develop and maintain strong working relationships with clinic management and staff members who provide standard care in outpatient clinic(s)
- Provide information in support of the NCI Cancer Center Support Grant, progress reports, renewal applications, other grant applications, and contract proposals
- Maintain a high level of knowledge regarding clinical research protocol development and conduct, budgeting, and contracts in order to serve as a resource for the staff and investigators
- Provide oversight of regulatory compliance and monitoring
- You can work independently, follow written instructions and able to work with a remote team
- Must be a University Student
- Ability to perform standard clinical laboratory tests, procedures and analyses accurately and with skill
- Knowledge of clinical protocols, procedures, and practices in specialty area
- Knowledge of Institutional Review Board (IRB), Biosafety and General Clinical Research Center (GCRC) applications
- Knowledge of proper safety precautions and procedures utilized in handling all types of laboratory specimens, reagents, chemicals and hazardous waste
- Report on activities of program to UACC administration, NCI, or others
- Work with the communications group to develop internal and external communications to develop positive and informative messaging for the UACC-Phoenix
- Support the UACC Director, Deputy Director, and Associate Director of Administration in driving unifying clinical development strategies across the two campuses, Tucson and Phoenix
- Support the Deputy Director and Medical Director of the UACC-Phoenix in the day-to-day administrative operations of the UACC-Phoenix, including hiring, grants, space, and clinical trials
- Develop and maintain Clinical Trials Office and other administrative policies, procedures, work instructions, training programs, and other resource information that allows the staff to perform efficiently and to deliver a high quality work product
- A High or Secondary School diploma/certificate or country’s educational equivalent (MBO/HBO)
- Develop and manage of a research matrix to monitor the progress of all Clinical Trials within the Division of Cardiology
- Assess feasibility and ongoing monitoring of clinical trial workload across the Division of Cardiology with the Division’s Clinical Director of Research
- Assist the Clinical Trials Finance Manager for budget development for upcoming clinical trials
- Provide ongoing support for other research coordinators regarding UC Denver’s research processes and study-specific procedures
- Knowledge of research techniques
- Knowledge of use and care of laboratory equipment, instruments, and supplies
- Should be a University Student
- Bachelor's degree or equivalent with at least less than 1 year experience in administration
- Experience handling biological samples (blood and urine specimens)
- Experience executing protocol-required tasks
- Supervision, training and mentoring of PRAs, serving as clinical trial coordinators, in day to day processes and query resolutions
- Evaluate study assignments and assess work load
- Conduct periodic audits of all the study documentation to ensure compliance and completeness
- Work directly with industry sponsor monitors to ensure completeness of all work submitted
- Troubleshoot database resolution
- Monitor and track data entry, subject screening and enrollment activities, and troubleshoot areas where needed for compliance
- Develop and submit accurate IRB applications, Continuing Review Reports and all other required documents to support the conduct of clinical trials research
- Formulate consent documents in accordance with IRB regulations and sponsor-specific requests
- Assist with the drafting of protocols, case report forms, and other required documents for Investigator Initiated trials
- Provide direction and support for subject recruitment, tracking of study visit schedule appointments
- Knowledge of quality assurance methods and procedures as applicable
- Knowledge of cardiology patient populations and ability to perform telephone screenings
- Ability to elicit clinically significant information from patients/subjects
- Demonstrated ability to perform lab duties safely and ship laboratory specimens on time
- Ability to prepare IRB forms
- Bachelor’s degree in a related field (Business Administration, Nursing, Healthcare Administration, Clinical Research Management, ) and at least 5 (five) years of work experience in a clinical research setting
Related Job Descriptions
Create a Resume in Minutes with Professional Resume Templates
I am an Employer
I am a candidate.
Clinical Research Assistant
Job summary.
The Division of Urologic Oncology within the Department of Urology is currently seeking a highly motivated individual to help assist physicians and patients in the clinical trial enrollment process. The primary goals for this position are to increase both physician and patient engagement with clinical trials and provide more patients with the opportunity to enroll in ongoing trials related to urologic oncology .
Responsibilities*
The Clinical Research Assistant will screen patients across multiple urology clinics with all available urologic oncology clinical trials in mind. This candidate will notify the coordinators and providers of the studies that the patients qualify for, including if there is more than one option available. This person will help coordinators and providers to prioritize the clinical trials that patients may be eligible for. The CRA will also assist outside patient referrals with coordinating appointments and pre-screening for clinical trials. - 50%
The Clinical Research Assistant will at times also act as a liaison with the radiation oncology clinic and medical oncology clinics when clinical trials are shared between departments and be the point person for providers and coordinators to reach out to for the clinical trial contacts. - 10%
The Clinical Research Assistant will maintain an up-to-date list of studies and study contacts as well as making sure clinical trial webpages are up to date with the most current study/protocol information. This will also include helping to develop tools to easily identify key eligibility for trials. - 30%
The CRA will also be available to answer some questions from patients regarding study logistics and direct them to the appropriate section of the consent document. The CRA will also help disseminate trial availability to potentially eligible patients, to the extent permitted according to each study's IRB approval. - 5%
The CRA will also update urologic oncology faculty with their individual recruitment successes and those of their peers. Although this position would not be enrolling patients to the studies as their main role, there may be times study coordinators may need in-clinic assistance for study enrollment. Additionally, the CRA will at times assist in ensuring proper completion and management of source documents, including consent. - 5%
Supervision Received
This position receives direct supervision and reports directly to the Project Coordinator and Senior Project Manager.
Required Qualifications*
- High school diploma or GED is necessary.
- Personable, empathetic, and able to communicate effectively
- Excellent organizational skills and can prioritize competing tasks to meet deadlines
- Able to work independently for extended periods of time while also being a team player and maintaining effective communication with the study coordinator, senior project manager and principal investigators.
Work Schedule
This position is hybrid with being on site 3-days per week.
Modes of Work
Positions that are eligible for hybrid or mobile/remote work mode are at the discretion of the hiring department. Work agreements are reviewed annually at a minimum and are subject to change at any time, and for any reason, throughout the course of employment. Learn more about the work modes .
Additional Information
Michigan Medicine is firmly committed to advancing inclusion, diversity, equity, accessibility, and belonging, which are core to the culture and values of the Medical School Office of Research. Our community supports recruiting and cultivating a diverse workforce as a reflection of our commitment to serve the diverse people of Michigan and the world. We strive to create a work culture where each team member feels respected, valued, and safe.
Background Screening
Michigan Medicine conducts background screening and pre-employment drug testing on job candidates upon acceptance of a contingent job offer and may use a third party administrator to conduct background screenings. Background screenings are performed in compliance with the Fair Credit Report Act. Pre-employment drug testing applies to all selected candidates, including new or additional faculty and staff appointments, as well as transfers from other U-M campuses.
Application Deadline
Job openings are posted for a minimum of seven calendar days. The review and selection process may begin as early as the eighth day after posting. This opening may be removed from posting boards and filled anytime after the minimum posting period has ended.
U-M EEO/AA Statement
The University of Michigan is an equal opportunity/affirmative action employer.

How to Become a Clinical Research Assistant: A Complete Guide to Becoming A CTA with No Experience on Resume

How To Become A Clinical Research Assistant
A complete guide to becoming a clinical trial assistant with no experience on resume.
Clinical Research Assistant
The work of a clinical trial/research assistant (CTA) in clinical research can never be overstated. It is an important career that requires a lot of interest and dedication in order to be successful. If you have developed interest in becoming a CTA, there are certain questions that you must ask yourself. Are you really cut out for this career path? Are you eager to take up more responsibilities in a work place? Can you monitor the trial subject and ensure that the trial is conducted in a safe and ethical manner? If your answers to this questions are yes, then you might just be cut out for the job of a clinical trial assistant.
CCRPS offers the only accredited 5-day, on-demand advanced clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Responsibilities of a Clinical Research Assistant
A clinical trial assistant have a lot of responsibilities and roles to fulfill within a clinical research institute to ensure the success of a project. Some of these responsibilities include:
Maintaining the standard operating procedures (SOP).
Provide regular report updates of the progress of clinical studies to the appropriate personnel.
Planning and conducting of pre-study site evaluation.
Conduct clinical site feasibility and are as well involved in study visibility.
Assess the study subjects to ensure that the appropriate clinical protocols are observed and the trial is in sync with laid down regulations.
Research Assistant Job Description
Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives.
May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related area. Knowledge of FDA regulatory requirements is required. Has knowledge of commonly-used concepts, practices and procedures within a particular field. Rely on instructions and pre-established guidelines to perform the functions of the job. Work under immediate supervision. Primary job functions do not typically require exercising independent judgment. Typically reports to a supervisor or manager.
Minimum Education Requirements For Clinical Trial Assistant
Requirements:
Completed degree(s) from an accredited institution that are above the minimum education requirement may be substituted for experience on a year for year basis
High school diploma or equivalent; college degree preferred
The educational requirement for a clinical research assistant is at the very least a high school diploma or associate degree in a health science. That's the least requirement, although more employers now prefer a B.Sc degree. Even if you don’t have a health science degree. if you took sciences related courses like nursing, life sciences, medical science, biotechnology, you should absolutely let the companies you are applying to know.
Another avenue you can become a clinical trial assistant is through certification. This is possible and is most common for people without formal education in the fields mentioned. Certification can be very demanding as it requires a lot of administrative knowledge in the area of clinical research. Many CTAs move on to become CRCs, CRAs, and administrators.
Skills You Need To Show On Your Research Assistant Resume
To be successful as a clinical research assistant, there are certain skill sets that are required.
A knowledge of the challenges and restrictions involved in the implementation and retention of databases.
A complete understanding of the responsibilities and liabilities involved in the use of humans for trial tests.
An ability to make excellent clinical development plan.
Must be able to ensure that data gotten from clinical trials are accurate and reliable and the legal rights and privacy of the subjects are protected.
Having these above listed skills and being efficient in them make the job of a clinical trial assistant easier and more interesting. Responsibilities:
Conduct literature reviews
Collect and analyze data
Prepare materials for submission to granting agencies and foundations
Prepare interview questions
Recruit and/or interview subjects
Maintain accurate records of interviews, safeguarding the confidentiality of subjects, as necessary
Summarize interviews
Provide ready access to all experimental data for the faculty researcher and/or supervisor
Request or acquire equipment or supplies necessary for the project
Manage and respond to project related email
Prepare, maintain and update website materials
Supervise undergraduate students working on the research project (maintaining records on assignment completion, acting as liaison/mediator between the undergraduate students and the faculty researcher)
Attend project meetings
Attend area seminars and other meetings as necessary
Summarize project results
Prepare progress reports
Prepare other articles, reports and presentations
Monitor the project budget
Travel to field sites to collect and record data and/or samples as appropriate to the specific objectives of the study
As appropriate to the specified position, code and verify data in accordance with specified research protocol and coding procedures and enter data into a computer database and/or spreadsheet application for subsequent analysis
Develop or assist in the development of interview schedules; contact potential subjects to introduce and explain study objectives and protocol and to arrange interviews, either in person or by telephone
Identify and compile lists of potential research subjects in accordance with study objectives and parameters, as appropriate to the individual position
Conduct and record face-to-face and/or telephone interviews with subjects, in accordance with predetermined interview protocol, data collection procedures and documentation standards
Review and edit data to ensure completeness and accuracy of information; follow up with subjects to resolve problems or clarify data collected
May set up, calibrate and maintain laboratory and/or field research equipment, as specified by the requirements of the study
May lead or guide the work of student employees
Perform miscellaneous job-related duties as assigned
Prepare findings for publication and assist in laboratory analysis, quality control, or data management
Write and contribute to publications
Develop research protocols
Track progress over time
Assist with preparation of all educational and training workshops and evaluation strategies
Engage clinical and community partners in research
Market training and technical assistance resources to clinical partners and academic investigators
Develop assessment and evaluation tools
Compile data for progress reports
Where To Reach Out For Trial Assistant Experiences And Internships
Landing that first trial assistant experience or internship can be a stepping stone to a rewarding career in clinical research. In this blog, we'll explore various avenues to find these valuable opportunities and launch your journey in this dynamic field.
Education and Certification:
While not always mandatory, a degree in a life sciences field like biology, health sciences, or nursing can be beneficial. However, even without a degree, you can break into the field. Consider pursuing a certification program offered by organizations like the Association of Clinical Research Professionals (ACRP) to demonstrate your knowledge and commitment.
Finding Trial Assistant Opportunities:
Industry-Specific Platforms: Leverage job boards frequented by the clinical research community. Look for platforms like Society for Clinical Research Associates (SOCRA) or ACRP job boards. These boards often list internship and entry-level positions specifically for Clinical Trial Assistants (CTAs).
General Job Boards: Don't neglect popular job boards like Indeed or LinkedIn. Utilize relevant keywords like "clinical trial assistant internship" or "research assistant" to filter your search and uncover a wider range of opportunities.
University Resources:
Career Services Departments: Many universities have dedicated career centers that assist students in finding internships. Connect with your career advisor to discuss your interest in clinical research and explore internship opportunities within the university or with partnering institutions.
Research Departments: Universities often conduct their own clinical trials. Reach out to professors or research departments to inquire about potential research assistant positions. This can provide valuable hands-on experience.
Government Websites:
Regulatory Agencies: The US Food and Drug Administration (FDA) offers student volunteer programs ([resources for getting experience in clinical research]).
Networking:
Professional Associations: Join associations like ACRP or SOCRA. Attend industry conferences or webinars to connect with professionals, learn about the field, and discover potential internship or job openings.
LinkedIn: Build your professional profile on LinkedIn and connect with individuals working in clinical research. Reach out to them politely and express your interest in gaining experience. Show genuine curiosity and highlight your transferable skills.
Local Directories:
CTAs can leverage online directories to target their search. After obtaining your certification, reach out to request experiences or internships at:
Clinical research organizations (CROs)
Pharmaceutical companies
Biotechnology companies
These directories can be found through professional association websites or a simple online search using terms like "USA clinical trial directory" or "USA CRO directory". Here are some examples:
ClinicalTrials.gov (a comprehensive listing of clinical trials registered in the US)
CRO Directory (searchable directory of contract research organizations)
BioPharmCatalyst (industry resource with listings of clinical trials and CROs)
Volunteering:
Hospitals and Research Institutions: Volunteer at hospitals or research institutions involved in clinical trials. This can provide valuable firsthand experience and build connections with professionals in the field.
Additional Tips:
Tailor Your Resume and Cover Letter: Highlight relevant coursework, volunteer experiences, and any transferable skills that demonstrate your aptitude for the role. Research the specific company or institution you're applying to and tailor your application to their needs.
Be Proactive: Don't wait for opportunities to come to you. Research companies conducting trials in your area and directly contact their clinical research departments. Express your enthusiasm and willingness to learn.
By exploring these avenues and demonstrating your enthusiasm, you'll increase your chances of landing a trial assistant experience or internship and taking that crucial first step towards a fulfilling career in clinical research.
Clinical Trial Assistant Training
Unlike the hundreds of CTAs who apply to a position, you can give your resume and interview a huge advantage by having certification. CCRPS' offers complete clinical trial assistant training and certification by the ACCRE through our clinical trial assistant training course. Certification as a CTA can help you show competency to work and apply for roles; many students use the course as a way to update their resume and land the interview at the site they desire. If you plan to continue a career in clinical research, ask our 24/7 chat and phone advisors for partial scholarships. We also offer up to 4 month payment plans ($100 per month).
Advanced Clinical Trial Assistant Training: CTA Syllabus CCRPS
DEMO COURSE
Introduction
Accreditation Council For Clinical Research & Education for CCRPS
Fundamentals Of Clinical Research
An Introduction to Clinical Research
An Overview of ICH GCP
Code of Federal Regulations
CFR 21 Part 11
Clinical Trial Roles And Responsibilities
Sponsor/CRO Responsibilities
13 Principles, IRB, & Investigator Roles
Informed Consent & Patient Safety
Informed Consent FREE PREVIEW
Safety of Human Subjects in Clinical Research FREE PREVIEW
Adverse Event Reporting & Responsibilities
Reporting Responsibilities of the Investigators
Adverse Events
Ethical Research In Vulnerable Populations
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated FREE PREVIEW
Ethics of Research Involving Pregnant Women and Fetuses
Ethics of Research Involving Prisoners
Trial Management, Data Handling, And Record Retention
Trial Management – Data Handling and Record Retention
a) Common Terminology Used In Clinical Research
b) Commonly Used Abbreviations and Terms in Clinical Research
Clinical Trials - Advanced Review
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
FREE PREVIEW
Subject Recruitment, Retention, And Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Misconduct And Fraud
Scientific Misconduct and Fraud
Detecting Falsification
Clinical Trial Assistant Certification Exam
ICH GCP Clinical Trials Assistant Exam (30 Questions)
What To Know For Clinical Trial Assistant Interview Questions
The work of a clinical research assistant is one of extreme importance to the clinical research institute, and employers will to testing to see if you understand what position entails.
Clinical research assistants is to test new medications, therapies and types of treatment and new medical devices to be sure of the safety of their use and the efficacy or efficiency of their work. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden. That is where clinical research assistants come in.
Clinical research assistant are responsible for performing the different safety and quality checks within their unit. Some of these checks are routinely carried out daily, weekly or monthly. The daily checks are usually the first thing they carry out on resuming to work everyday. This is to ensure the safety of all the staffs and volunteers within the clinical research institute and as well improve the quality of the data collected and the results.
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, they are responsible for collecting and analyzing the data gotten from clinical tests and trials and they also evaluate the result. They are the ones that keep all the record of activities in the clinical research institute for the purpose of references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The standard equipment like freezers and fridges are checked at least twice daily. This is important because they are used for storing specific research samples and other medications that needs to be kept in controlled temperature and a slight deviation from that can affect the validity of the result and the research. The emergency equipment are also checked regularly on a daily basis.
Part of a clinical research assistant's job is to assist all members of the team and deal with different queries from members of the public. It is also their duty to control all medical stock used in their unit, prepare materials for screening visits, prepare consent forms, questionnaires and information sheets and keeping study files while archiving the files for completed studies.
In the midst of these many duties, it is very important that the clinical research assistant is very capable of multitasking. A good communication skill (both written and verbal) is very important to do this work successfully. One thing that is a must for anyone aspiring to take up this job is to have a keen eye for details. It is also important to be able to ask the right question and develop your knowledge base as much as possible. If you can demonstrate that you have these skills in your interview, you should be all set to go.
Clinical Research Assistant Salary

Per Payscale
The salary of a clinical research assistant can vary depending on different factors like location, institution or employer etc. However, the average yearly salary is $41,000 at an hourly average of $17. It can rise as high as $55,000 or as low as $32,000.
If you'll like to apply for the post of a clinical research assistant, it makes it easier for you if you have B.Sc in life science or social science related courses. If you don't, go enroll for a bachelor's degree and get experiences by volunteering in clinical trials
The purpose of clinical research is to test new medications, therapies, and new medical devices to be sure of the safety of their use and their efficiency. These clinical trials are very much regulated and seriously monitored to ensure that they comply with the laid down regulations. The need to keep various records, in order to meet up with compliance requirements can be a burden.
That is where clinical research assistants come in….
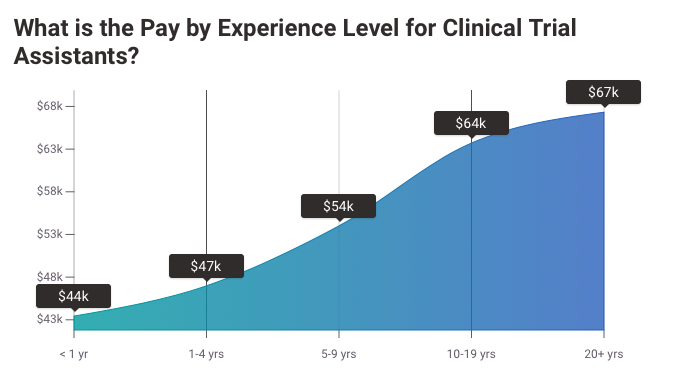
CTA Salary Prediction Provided by Payscale
CCRPS offers the only clinical research assistant certification (ACRAC) course available
The job of a clinical research assistant is to help in finding subjects that can be used for clinical trials, collecting and analyzing the data from clinical tests, and they also evaluate the result.
They are the ones who keep all the record of activities in the clinical research institute for future references. They practically ensure that the clinical trial activities are in line with laid down regulations. The amount of data to be collected, evaluated and stored form the trials make this job an important one. That means it is a job in high demand.
Their importance means that there are a variety of places where they can work. Clinical research assistants can work at clinical research institutes, medical centers, pharmaceutical companies, biotech companies and a whole host of other medically and clinically inclined organizations.
The educational requirements required to work as a clinical research assistant includes a bachelor’s degree, master’s degree or a doctorate degree in life sciences or other medical related sciences.
These are just basic educational requirements, if you are interested in getting into clinical research, you need more than just degrees in life science. Not because they are not important but because they do not offer you the core knowledge and experience needed to be successful in this career. Based on your chosen discipline in clinical research, you can choose to offer courses related to your discipline and you will be taught by seasoned and experience lecturers in the industry keen to pass on their knowledge and experience. You can also register to be a member of clinical research based associations at CCRPS and find more expert information on the clinical research field. All you need to have a rapid career is right here.
CCRPS offers the only accredited 5-day, on-demand clinical research assistant certification (ACRAC) course available to help your learn and apply knowledge and increase your chances of 1) getting a job 2) being efficient and successful in your career.
Principal Investigator Training - Role of Principal Investigator in Clinical Research
Pharmacovigilance: a complete guide to pharmacovigilance and drug safety training.

IMAGES
COMMENTS
Clinical trial assistants provide support to research teams by coordinating and managing aspects of clinical trials. This post provides detailed information on the clinical trial assistant job description, including the key duties, tasks, and responsibilities they commonly perform.
Responsibilities. Assist in the design, administration, and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. Prepare and present detailed reports on the progress of ongoing research. Assist in the preparation of manuscripts for publication.
Clinical research assistants work in hospitals, laboratories, and other institutions that conduct scientific studies. They identify subjects or clinical trials, collect data, evaluate results, monitor clinical trials, and take notes on activities. They audit research trials and ensure all clinical trial protocols are in compliance.
A Clinical Trial Assistant earns an average salary of $48,787 (USD) per year. However, this salary can fluctuate based on factors such as level of experience, educational qualifications, and the location of the job. Additionally, the size and type of the organization can also have an impact on the salary.
Clinical Trial Assistant roles and responsibilities cover a range of tasks in support of a clinical study. These can include: submission of clinical trial documents for review and approval by the Ethics Committee. A CTA job description may describe the position as a 'Global Clinical Trial Assistant' or 'Clinical Trial Assistant Remote ...
Clinical research assistants work in hospitals, laboratories, and other institutions that conduct scientific studies. They identify subjects or clinical trials, collect data, evaluate results, monitor clinical trials, and take notes on activities. They audit research trials and ensure all clinical trial protocols are in compliance.
As a clinical research assistant, your responsibilities include preparing the laboratory, processing volunteers, taking biological samples or vital signs, and organizing data. You may also be required to set up and clean work areas. Your job is to assist the researchers in any way possible, helping them conduct sound, ethical, and ...
Responsibilities for clinical research assistant. Assisting the clinical team with administrative activities as required. Assisting Clinical Manager with monitoring tasks as required. Administer questionnaires, and assists with procedures for obtaining patient screening and recruitment obtaining research specimens.
We have included clinical trial assistant job description templates that you can modify and use. Sample responsibilities for this position include: Perform and/or maintain data entry in internal Quality systems. Prepare investigator budget payments and tracking systems. Plan and conduct Therapeutic Area meetings.
As a clinical research assistant, you will be responsible for conducting research studies, collecting and analyzing data, and working closely with medical professionals to ensure the safety and efficacy of new treatments. While attention to detail and strong organizational skills are essential, the job also requires a passion for helping others ...
The Clinical Trial Assistant job description template is a document that outlines the essential duties and responsibilities of a clinical trial assistant. This position supports clinical research studies to ensure proper documentation, protocol compliance, and study organization. The template can be used by hiring managers to create a detailed ...
Research Assistant Job Description. Participate in the design, administration, and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related ...
The duties and responsibilities of a Clinical Trials Assistant include: Assisting in the coordination and administration of clinical trials research studies. Collecting, transcribing and maintaining clinical trial data and patient information. Preparing and maintaining documentation and reports for clinical trials.
Clinical Trial Assistant Job Description. Clinical trials are designed to test new medications and medical devices for safety and efficacy. Trials are highly regulated and thoroughly monitored, which results in a significant record-keeping and compliance burden. Many organizations running trials hire clinical trial assistants as support staff.
Job Description / Roles and Responsibilities for Job Title: The primary responsibility of the Clinical Research Assistant | Clinical Trial Assistant is to perform basic assigned study tasks, to interact with study participants, and assist the Clinical
A clinical research assistant, CRA, is responsible for providing quality patient care and service to the clinical research clients and the healthcare providers. The clinical research assistant job description involves working closely with doctors, nurses, and other technicians in performing clinical and laboratory procedures.
Assistant Clinical Research Coordinator. University of North Carolina at Chapel Hill. Hybrid work in Chapel Hill, NC 27599. $45,000 - $53,000 a year. Full-time. Monday to Friday. Prefer candidate who Is familiar with clinical trials systems at UNC and Implementing research studies at UNC; Proficient in Spanish.
A competent Clinical Research Associate should be able to perform various duties and responsibilities. Proper fulfillment of a Clinical Research Associate's duties and responsibilities brings success to your company. Clinical Research Associates should assist in organizing and monitoring the different stages of clinical trials.
Marietta, GA 30060. $26 - $30 an hour. Full-time. 40 hours per week. Monday to Friday. Easily apply. Under the direction of the Clinical Research Manager, the Coordinator organizes research information for various clinical projects and trials.
Clinical Research Assistant Duties and Responsibilities. Monitor clinical trials and take notes on activities. Ensure compliance with all clinical trial protocols. Enter clinical research data into appropriate fields. Transfer data from paper formats via computer, recorders, or scanners. Organize spreadsheets with large numbers.
Responsibilities for clinical trials assistant. Contribute to, and participate in, study dry runs, pre-study planning activities, departmental and project specific meetings. Prepare and send CDAs, and other study related materials to clinical study sites. Coordinate in-house blood draws and internal sample collection studies.
A clinical trial assistant, or CTA, works within a clinical operations team to help them organise and complete successful pharmaceutical studies. They work in several scientific research settings, such as clinical research organisations, pharmaceutical companies or clinical research units organised at hospitals and universities.
Alpha Clinical Research Centers LLC 5.0. Orange City, FL 32763. Typically responds within 5 days. $40,000 - $50,000 a year. Full-time. Monday to Friday + 2. Easily apply. Attends pertinent educational or study activities and reviews current literature relevant to clinical trials assigned.
The Clinical Research Assistant will maintain an up-to-date list of studies and study contacts as well as making sure clinical trial webpages are up to date with the most current study/protocol information. This will also include helping to develop tools to easily identify key eligibility for trials. - 30%
Research Assistant Job Description. Participate in the design, administration and monitoring of clinical trials. Analyze and evaluate clinical data gathered during research. Ensure compliance with protocol and overall clinical objectives. May require a BS, RN, or BSN degree or equivalent and 0-3 years of experience in the field or in a related ...