An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Novel Insights Into the Genetic Causes of Short Stature in Children
Affiliation.
- 1 Department of Paediatrics, University of Chieti, Chieti, Italy.
- PMID: 35949366
- PMCID: PMC9354945
- DOI: 10.17925/EE.2022.18.1.49
Short stature is a common reason for consulting a growth specialist during childhood. Normal height is a polygenic trait involving a complex interaction between hormonal, nutritional and psychosocial components. Genetic factors are becoming very important in the understanding of short stature. After exclusion of the most frequent causes of growth failure, clinicians need to evaluate whether a genetic cause might be taken into consideration. In fact, genetic causes of short stature are probably misdiagnosed during clinical practice and the underlying cause of short stature frequently remains unknown, thus classifying children as having idiopathic short stature (ISS). However, over the past decade, novel genetic techniques have led to the discovery of novel genes associated with linear growth and thus to the ability to define new possible aetiologies of short stature. In fact, thanks to the newer genetic advances, it is possible to properly re-classify about 25-40% of children previously diagnosed with ISS. The purpose of this article is to describe the main monogenic causes of short stature, which, thanks to advances in molecular genetics, are assuming an increasingly important role in the clinical approach to short children.
Keywords: Short stature; children; genetic cause; growth hormone.
© Touch Medical Media 2022.
PubMed Disclaimer
Conflict of interest statement
Disclosures: Concetta Mastromauro and Francesco Chiarelli have no financial or non-financial relationships or activities to declare in relation to this article.
Figure 1:. Genetic defects of growth hormone–insulin-like…
Figure 1:. Genetic defects of growth hormone–insulin-like growth factor-1 axis
Figure 2:. Genetic defects of growth plate…
Figure 2:. Genetic defects of growth plate paracrine factors
Similar articles
- Growth Hormone Treatment for Idiopathic Short Stature. Cutfield WS, Albert BB. Cutfield WS, et al. Pediatr Endocrinol Rev. 2018 Sep;16(Suppl 1):113-122. doi: 10.17458/per.vol16.2018.ca.ghidiopathicshortstature. Pediatr Endocrinol Rev. 2018. PMID: 30378789
- Application of Chromosomal Microarray for Evaluation of Idiopathic Short Stature in Asian Indian Children: A Pilot Study. Singh H, Tiwari P, Bhavi V, Chaudhary PS, Suravajhala P, Mohan MK, Mathur SK. Singh H, et al. Indian J Endocrinol Metab. 2018 Jan-Feb;22(1):100-106. doi: 10.4103/ijem.IJEM_202_17. Indian J Endocrinol Metab. 2018. PMID: 29535946 Free PMC article.
- Genetic evaluation in children with short stature. Zhou E, Hauser BR, Jee YH. Zhou E, et al. Curr Opin Pediatr. 2021 Aug 1;33(4):458-463. doi: 10.1097/MOP.0000000000001033. Curr Opin Pediatr. 2021. PMID: 34101704 Free PMC article. Review.
- Familial Short Stature. Rajkumar V, Waseem M. Rajkumar V, et al. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 32644549 Free Books & Documents.
- [Idiopathic short stature. A literature review and update]. Carrascosa A, Fernández Longás A, Gracia Bouthelier R, López Siguero JP, Pombo Arias M, Yturriaga R; Grupo Español de Consenso. Carrascosa A, et al. An Pediatr (Barc). 2011 Sep;75(3):204.e1-11. doi: 10.1016/j.anpedi.2011.05.007. An Pediatr (Barc). 2011. PMID: 21723798 Review. Spanish.
- Real-life long-term efficacy and safety of recombinant human growth hormone therapy in children with short stature homeobox-containing deficiency. Bruzzi P, Vannelli S, Scarano E, Di Iorgi N, Parpagnoli M, Salerno M, Pitea M, Elisabeth Street M, Secco A, Andrea Trettene A, Wasniewska M, Corciulo N, Tornese G, Felicia Faienza M, Delvecchio M, Filomena Madeo S, Iughetti L. Bruzzi P, et al. Endocr Connect. 2023 Jun 8;12(7):e220402. doi: 10.1530/EC-22-0402. Print 2023 Jul 1. Endocr Connect. 2023. PMID: 37014306 Free PMC article.
- Vasques GA, Andrade NLM, Jorge AAL. Genetic causes of isolated short stature. Arch Endocrinol Metab. 2019;63:70–8. - PMC - PubMed
- Silventoinen K. Determinants of variation in adult body height. J Biosoc Sci. 2003;35:263–85. - PubMed
- Wood AR, Esko T, Yang J. et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–86. - PMC - PubMed
- Faienza MF, Chiarito M, Brunetti G. et al. Growth plate gene involvement and isolated short stature. Endocrine. 2021;71:28–34. - PubMed
- Cohen P, Rogol AD, Deal CL. et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93:4210–17. - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding, linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central
Miscellaneous
- NCI CPTAC Assay Portal
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Website maintenance is scheduled for Saturday, September 7, and Sunday, September 8. Short disruptions may occur during these days.

CRAIG BARSTOW, MD, AND CAITLYN RERUCHA, MD
Am Fam Physician. 2015;92(1):43-50
Author disclosure: No relevant financial affiliations.
Short stature is defined as a height more than two standard deviations below the mean for age (less than the 3rd percentile). Tall stature is defined as a height more than two standard deviations above the mean for age (greater than the 97th percentile). The initial evaluation of short and tall stature should include a history and physical examination, accurate serial measurements, and determination of growth velocity, midparental height, and bone age. Common normal variants of short stature are familial short stature, constitutional delay of growth and puberty, and idiopathic short stature. Pathologic causes of short stature include chronic diseases; growth hormone deficiency; and genetic disorders, such as Turner syndrome. Tall stature has the same prevalence as short stature, but it is a much less common reason for referral to subspecialty care. Common causes of tall stature include familial tall stature, obesity, Klinefelter syndrome, Marfan syndrome, and precocious puberty. Although most children with short or tall stature have variants of normal growth, children who are more than three standard deviations from the mean for age are more likely to have underlying pathology. Evaluation for pathologic etiologies is guided by history and physical examination findings.
Most children with short or tall stature have normal variants of growth. The evaluation of potential pathologic causes of short or tall stature should be guided by the history and physical examination findings. 1 – 3
| History and physical examination findings should guide further evaluation for pathologic causes of short and tall stature. | C | – |
| World Health Organization growth charts should be used for children younger than two years, and the Centers for Disease Control and Prevention growth charts should be used for children older than two years. | C | |
| Midparental height growth velocity should be calculated to evaluate a child's growth vs. potential height. | C | – |
| Bone age should be compared with chronologic age to help narrow the differential diagnosis of short or tall stature. | C | , , |
Evaluation of Growth
The first step in the evaluation of a child with suspected short or tall stature is to obtain accurate measurements and plot them on the appropriate growth chart. For infants and toddlers, weight, length, and head circumference should be plotted on a growth curve at every visit. For patients two to 20 years of age, weight, height, and body mass index should be plotted. Length should be measured using a horizontal rule in children younger than two years, and height should be measured using a wall-mounted stadiometer in children older than two years. Because children grow in spurts, two measurements at least three to six months apart, and preferably six to 12 months apart, are needed to accurately determine growth velocity. 4
The Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics recommend using the World Health Organization (WHO) growth charts for children younger than two years and the CDC growth charts for children older than two years. 5 The CDC growth charts are a population-based reference that include data from bottle-fed and breastfed infants. Because the WHO growth charts are based on an international study of exclusively breastfed infants raised in optimal nutritional conditions, they are less likely to incorrectly identify breastfed infants as underweight. The CDC and WHO growth charts are available at http://www.cdc.gov/growthcharts/ and http://www.who.int/childgrowth/standards/en/ .
A newborn's size and growth are a result of the intrauterine environment, and growth hormone does not play a major role. Between six and 18 months of age, children exhibit catch-up or catch-down growth until they reach their genetically determined growth curve based on midparental height. By two years of age, growth hormone plays a predominant role. At this stage, children should track along a percentile, and variation should stay within two large bands on the growth chart. In adolescence, growth is affected by the onset of puberty, and sex hormones become the predominant factor in growth.
Variation from this normal pattern of growth may be a sign of pathologic conditions. Although most children with short or tall stature do not have a pathologic condition, extremes of height, especially beyond three standard deviations, require further workup.
MIDPARENTAL AND PROJECTED HEIGHT
Calculating the midparental height ( Table 1 ) is an important part of the evaluation because most short or tall children have short or tall parents. Projected height can be estimated by projecting the current growth curve to adulthood in children with normal bone age, or by using a bone age atlas in those with delayed bone age. Most children will have a projected adult height within 10 cm (4 in), or two standard deviations, of their midparental height. A projected height that differs from the midparental height by more than 10 cm suggests a possible pathologic condition. Short or tall parents may themselves have a pathologic reason for their height, especially if they are more than two standard deviations from the adult norm. 6 – 8
| [Paternal height (cm) – 13 cm + maternal height (cm)] ÷ 2 | |
| [Paternal height (in) – 5 in + maternal height (in)] ÷ 2 | |
| [Paternal height (cm) + 13 cm + maternal height (cm)] ÷ 2 | |
| [Paternal height (in) + 5 in + maternal height (in)] ÷ 2 |
Growth velocity is a measurement of growth rate. Children with normal variants of height tend to have a normal growth velocity (5 cm [2 in] per year for children between five years of age and puberty) after catch-up or catch-down growth. A growth velocity that is less than normal should prompt further investigation. Table 2 includes normal growth velocity by age. 1 , 9
| Birth to 12 months | 23 to 27 cm (9.06 to 10.63 in) |
| 12 months to 1 year | 10 to 14 cm (3.94 to 5.51 in) |
| 2 to 3 years | 8 cm (3.15 in) |
| 3 to 5 years | 7 cm (2.76 in) |
| 5 years to puberty | 5 to 6 cm (1.97 to 2.36 in) |
| Puberty | Girls: 8 to 12 cm (3.15 to 4.72 in) |
| Boys: 10 to 14 cm (3.94 to 5.51 in) |
Bone age should be compared with chronologic age to narrow the differential diagnosis of short stature. 5 , 10 , 11 The traditional method compares a plain radiograph of the left wrist and hand to a database of norms, although various methods are now available. 10 – 12 Children with normal variations of growth may have advanced or delayed bone age, but a bone age that is more than two standard deviations from the mean for age is likely due to a pathologic condition.
Short Stature
Short stature is defined as a height more than two standard deviations below the mean for age, or less than the 3rd percentile. Idiopathic short stature is defined as a height less than two standard deviations below the mean for age without a known etiology.
Most children with short stature have normal variants such as familial short stature, constitutional delay of growth and puberty, or idiopathic short stature. Approximately 5% of children referred for evaluation of short stature have an identifiable pathologic cause. 13 The most common etiologies are growth hormone deficiency, hypothyroidism, celiac disease, and Turner syndrome. Other causes include renal, hepatic, and gastrointestinal diseases, and other genetic syndromes. 10 – 15
The initial evaluation of short stature ( Figure 1 ) should include a history and physical examination, accurate growth assessment, calculation of the growth velocity and midparental height, and radiography to evaluate bone age. 16 Drugs known to cause short stature include steroids (chronic use), attention-deficit/hyperactivity disorder medications, and anticonvulsants. Dysmorphic characteristics suggest a genetic disorder, whereas midline defects suggest an abnormality of the growth hormone axis. Table 3 includes the differential diagnosis of short stature. 1 , 2 , 4 , 16 – 18
| Constitutional delay of growth and puberty | Normal growth velocity, history of delayed puberty in parents | History and physical examination, bone age |
| Familial short stature | Short parents, projected height consistent with midparental height, normal growth velocity | Midparental height, growth velocity, bone age; consider targeted laboratory evaluation |
| Idiopathic short stature | Height < 2 standard deviations below the mean for age with no identified pathology, normal growth velocity and bone age | Laboratory testing to rule out pathology |
| Anemia | Fatigue, pallor, poor growth | Complete blood count |
| Celiac disease | Abdominal pain, malabsorption, anemia; short stature may be the only symptom | Tissue transglutaminase and total immunoglobulin A measurements; consider referral for endoscopy and biopsy |
| Chronic renal insufficiency | History of renal disease, poor weight gain | Creatinine measurement, urinalysis |
| Inflammatory bowel disease | Abdominal pain, bloody stool, poor weight gain | Erythrocyte sedimentation rate and C-reactive protein measurements, referral for endoscopy and biopsy |
| Achondroplasia | Short limbs; long, narrow trunk; large head with prominent forehead | Skeletal radiography, testing for the gene |
| Acquired growth hormone deficiency | History of head trauma or cranial irradiation, central nervous system infection | IGF-1 and IGFBP-3 measurements, referral for growth hormone stimulation, other pituitary function tests |
| Congenital growth hormone deficiency | Hypoglycemia, birth length may be normal, height and bone age progressively delayed; jaundice, microphallus, midline craniofacial abnormalities | IGF-1 and IGFBP-3 measurements; referral for growth hormone stimulation, magnetic resonance imaging, other pituitary function tests |
| Congenital hypothyroidism | Mental retardation if not identified early | Newborn screening, thyroid-stimulating hormone and free thyroxine (T4) measurements |
| Intrauterine growth deficiency | Born small for gestational age, normal height not achieved by 2 to 4 years of age | Focused laboratory testing to evaluate organic causes, consider referral to pediatric endocrinologist |
| Primary nutritional deficiency | History of poor nutrition, weight loss precedes height loss | Nutritional history |
| Turner syndrome | Short stature, webbed neck, characteristic facies, short metacarpals, broad chest with widely spaced nipples, hyperconvex fingernails and toenails; may be normal appearing; decreased growth velocity and delayed puberty | Follicle-stimulating hormone, karyotyping |
If the initial evaluation suggests a genetic, endocrine, or gastrointestinal disorder, laboratory testing should be performed ( Table 4 ) . 1 , 3 , 13 , 14 , 16 , 19 , 20 In an asymptomatic child with short stature, an evaluation of the growth curve may provide clues to the underlying pathology. Underweight in a child with short stature suggests a systemic illness or malnutrition, whereas overweight suggests an endocrine disorder. 2 , 21
| Complete blood count | Anemia |
| Comprehensive metabolic panel | Hepatic and renal diseases |
| Erythrocyte sedimentation rate, C-reactive protein | Inflammatory bowel disease |
| Follicle-stimulating hormone, karyotyping | Turner syndrome |
| Insulinlike growth factor 1 | Growth hormone deficiency |
| Thyroid-stimulating hormone, free thyroxine (T4) | Hypothyroidism |
| Tissue transglutaminase and total immunoglobulin A | Celiac disease |
| Urinalysis | Renal disease |
| Insulinlike growth factor 1 | Growth hormone excess |
| Karyotyping | Klinefelter syndrome (XXY) |
| Serum luteinizing hormone, follicle-stimulating hormone, testosterone | Precocious puberty |
| Thyroid-stimulating hormone, free thyroxine (T4) | Hyperthyroidism |
Different causes of short stature tend to fall within identifiable growth patterns, and a review of a child's growth curve and bone age should guide further evaluation. Children with familial short stature or idiopathic short stature have a bone age equivalent to their chronologic age, and children with constitutional delay of growth and puberty or endocrine disorders have a bone age that is less than their chronologic age. Because the bone age of a child with endocrine diseases will progressively fall behind chronologic age, calculating bone age every 12 months might be useful to differentiate constitutional delay of growth from endocrine diseases. 1
Children with endocrine disorders, such as growth hormone deficiency, hypothyroidism, or glucocorticoid excess, have normal to increased weight, whereas children with systemic disease tend to have decreased height and weight. 2 , 21
If findings from the initial evaluation do not suggest a diagnosis, laboratory testing may be performed ( Table 4 ) . 1 , 3 , 13 , 14 , 16 , 19 , 20 A retrospective study found that a complete laboratory evaluation of an asymptomatic child with idiopathic short stature is low yield and expensive. The two diseases that were most often identified in the studied cohort were celiac disease and an abnormality of the growth hormone axis. 3 If history and physical examination findings do not suggest a cause, a complete blood count, comprehensive metabolic panel, and measurement of bone age, insulinlike growth factor 1, and insulinlike growth factor binding protein 3 might be useful to screen for chronic disease and growth hormone deficiency. Karyotyping in girls might also be reasonable because short stature and delayed puberty may be the only symptoms in some girls with Turner syndrome.
Children with short stature and no identified cause and children with certain other identifiable causes of short stature should be referred to a pediatric endocrinologist. Table 5 lists the indications for referral. 2 , 6 , 22
| Children with intrauterine growth retardation who do not catch up to the growth curve by 2 years of age |
| Height more than 3 standard deviations below the mean for age |
| Growth velocity < 5 cm (2 in) per year |
| No onset of puberty by 14 years of age for boys or 13 years of age for girls |
| Projected height more than 2 standard deviations (10 cm [4 in]) below the midparental height |
| Bone age more than 2 standard deviations below chronologic age |
| Diagnosis of conditions approved for recombinant growth hormone therapy |
SELECTED CAUSES
Constitutional Delay of Growth and Puberty . Children with this condition are born appropriate for gestational age, but will then fall to the 3rd percentile for height during catch-down growth. After this period, growth velocity will be normal and bone age delayed. 22 Children with this condition have delayed onset of puberty, resulting in a normal adult height.
Growth Hormone Deficiency . This condition may be congenital or acquired, and has an incidence of one in 3,000 to 9,000 children. 13 A history of head trauma, central nervous system infection, birth trauma, or cranial irradiation may suggest an acquired cause of growth hormone deficiency. Most infants with the congenital form are normal size at birth, but may have episodes of hypoglycemia or prolonged jaundice. Physical examination may reveal microphallus or midline craniofacial abnormalities. A child whose growth is initially normal but then falls progressively further off the growth curve may have growth hormone deficiency. Typically, children with this condition have a delayed bone age with a preserved or increased weight for age. The diagnosis can be made by a decreased insulinlike growth factor 1 or insulinlike growth factor binding protein 3, followed by negative growth hormone provocation test results. 23
Small for Gestational Age . Infants born small for gestational age typically have catch-up growth in the first 24 months, but 10% have a final height more than two standard deviations below the mean for age. 24 Children who do not have catch-up growth within the first six months or whose height is not within two standard deviations of the mean for age by two years of age may have a pathologic condition. It may take more than four years for a preterm infant who is born small for gestational age to attain a normal height. 24
Recombinant growth hormone is approved for a variety of conditions that cause short stature, including Turner syndrome, chronic renal failure, Prader-Willi syndrome, small for gestational age, Noonan syndrome, short stature homeobox-containing gene deficiency, and idiopathic short stature. It is administered through daily injections over several years. The injections are generally well tolerated, but rare adverse reactions have been reported. For children with idiopathic short stature, four years of treatment results in an increased height of 3.7 cm (1.46 in) and costs between $100,000 and $120,000. 25 , 26
Oxandrolone (Oxandrin) is an oral anabolic steroid that has been shown to increase height velocity but has little effect on final height. Insulinlike growth factor has been used in children with insulinlike growth factor deficiency. Although aromatase inhibitors have been used in children with idiopathic short stature, long-term effectiveness and safety data are not available. 27
Tall Stature
Tall stature is defined as a height more than two standard deviations above the mean for age (greater than the 97th percentile). Evaluation may also be needed in a child who has a normal height, but a projected height more than two standard deviations from the midparental height. Figure 2 is an algorithm for the evaluation of tall stature. 19 Although the percentage of children with tall stature is equal to that of children with short stature, children with tall stature are much less likely to be referred to subspecialty care.
Table 6 includes the differential diagnosis of tall stature. Constitutional advancement of growth in tall children is the equivalent of constitutional delay of growth and puberty in short children. 1 , 19 , 20 Children with constitutional advancement of growth have accelerated growth until two to four years of age and then track parallel to the growth curve. Puberty usually occurs early, leading to a near-normal height. 19
| Constitutional advancement of growth | Family history of early puberty, bone age greater than chronologic age | History and physical examination, bone age | |
| Familial tall stature | Projected height within 5 cm (2 in) of midparental height, bone age greater than chronologic age, normal growth velocity after catch-up growth | Growth velocity, bone age | |
| Hyperthyroidism | Rapid childhood growth, goiter, tachycardia, hypertension, diarrhea, fine tremor, exophthalmos | Thyroid-stimulating hormone and free thyroxine (T4) measurements | |
| Obesity | Body mass index greater than the 95th percentile, slightly early onset of puberty, modest overgrowth/tall stature, minimally advanced bone age | Bone age | |
| Pituitary gigantism (excess growth hormone) | Coarse facial features, mandibular prominence, broad root of nose, broad hands and feet, excessive sweating, hypertension, glucose intolerance | Measurement of insulinlike growth factor 1 and insulinlike growth factor binding protein 3, brain/pituitary magnetic resonance imaging, glucose suppression test | |
| Precocious puberty | |||
| Central | Girls: breast development before 8 years of age | Measurements of luteinizing hormone, follicle-stimulating hormone, estradiol, and testosterone | |
| Boys: testicular enlargement (> 3 mL) before 9 years of age | |||
| Peripheral | Same as the central form | Measurement of 17α-hydroxyprogesterone, human chorionic gonadotropin, dehydroepiandrosterone, estradiol, and testosterone; bone age | |
| Disproportionate overgrowth | |||
| Beckwith-Wiedemann syndrome | Macrocephaly, macroglossia, ear pits, renal abnormality, omphalocele, umbilical hernia, hepatosplenomegaly | Insulin and glucose measurements, advanced bone age, karyotyping, renal ultrasonography, echocardiography | |
| Homocystinuria | Marfan-like habitus, developmental delay, inferior subluxation of lens | Homocysteine and methionine measurements, dilated eye examination | |
| Klinefelter syndrome (XXY) | Delayed puberty; infertility; small, firm testes; gynecomastia; high-pitched voice; learning disability | Measurements of luteinizing hormone, follicle-stimulating hormone, and testosterone; karyotyping | |
| Marfan syndrome | Increased arm span, thin extremities, superior subluxation of lens, hypotonia, kyphoscoliosis, cardiac valvular deformities, aortic root dilation | Clinical diagnosis using Ghent criteria, testing for gene mutation, dilated eye examination | |
| Proportionate overgrowth | |||
| Fragile X syndrome | Large, protruding ears; long face; high-arched palate; hyperextensible fingers; pes planus; soft skin; macro-orchidism | Clinical suspicion based on dysmorphic features, testing for gene mutation | |
| Sotos syndrome (cerebral gigantism) | Large head; long, thin face; broad forehead; prominent, narrow jaw; downward slanting palpebral fissures; feeding difficulties from birth; facial flushing; hypotonia | Clinical suspicion based on dysmorphic features, renal ultrasonography, echocardiography, advanced bone age | |
| Weaver syndrome | Small chin, broad forehead, hypertelorism, long philtrum, camptodactyly | Clinical suspicion based on dysmorphic features, renal ultrasonography, brain magnetic resonance imaging, advanced bone age (from birth) | |
Obese children are tall for their age. 19 However, these children often have an early onset of puberty and therefore a near-normal final height. 20
Intervention is usually not needed in children with tall stature. High-dose sex steroids have been used to promote growth plate closure, but use has decreased over the past 20 years because of adverse effects. 28 Surgical destruction of the growth plates has also been performed, but this procedure is controversial. In patients with pituitary gigantism, octreotide (Sandostatin) and pegvisomant (Somavert) have been used to suppress the growth hormone. 19
Data Sources : We searched PubMed, Agency for Healthcare Research and Quality, Cochrane Database of Systematic Reviews, and National Guidelines Clearinghouse. Search terms included short stature, tall stature, and growth hormone. We did online searches of The New England Journal of Medicine , Pediatrics , American Family Physician , Pediatrics in Review , and the British Medical Journal to identify additional relevant articles. The bibliographies of review articles and textbook chapters were also reviewed for original research articles. The Pediatric Endocrine Society website was searched for consensus statements and clinical guidelines. Search dates: June and December 2014, and March 2015.
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Army Medical Department or the U.S. Army Service at large.
Nwosu BU, Lee MM. Evaluation of short and tall stature in children. Am Fam Physician. 2008;78(5):597-604.
Kaplowitz PB. Short stature. In: McInerny TK, ed. Textbook of Pediatric Care . Washington, DC: American Academy of Pediatrics; 2009:1727–1730.
Sisley S, Trujillo MV, Khoury J, Backeljauw P. Low incidence of pathology detection and high cost of screening in the evaluation of asymptomatic short children. J Pediatr. 2013;163(4):1045-1051.
Keane V. Assessment of growth. In: Kliegman R, Nelson WE, eds. Nelson Textbook of Pediatrics . 19th ed. Philadelphia, Pa.: Elsevier/Saunders; 2011.
Grummer-Strawn LM, Reinold C, Krebs NF Centers for Disease Control and Prevention. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States [published correction appears in MMWR Recomm Rep . 2010;59(36):1184]. MMWR Recomm Rep. 2010;59(RR-9):1-15.
Turner T, Yazdani P, French S. Endocrinology: short stature and growth. PediaLink: AAP Online Learning Center. http://pedialink.aap.org/home (subscription required). Accessed August 2014.
Cohen LE. Idiopathic short stature: a clinical review. JAMA. 2014;311(17):1787-1796.
Rose SR, Vogiatzi MG, Copeland KC. A general pediatric approach to evaluating a short child. Pediatr Rev. 2005;26(11):410-420.
Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317-329.
Cox LA. The biology of bone maturation and ageing. Acta Paediatr Suppl. 1997;423:107-108.
Cao F, Huang HK, Pietka E, Gilsanz V. Digital hand atlas and web-based bone age assessment: system design and implementation. Comput Med Imaging Graph. 2000;24(5):297-307.
Bilgili Y, Hizel S, Kara SA, Sanli C, Erdal HH, Altinok D. Accuracy of skeletal age assessment in children from birth to 6 years of age with the ultrasonographic version of the Greulich-Pyle atlas. J Ultrasound Med. 2003;22(7):683-690.
Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29-35.
Grote FK, Oostdijk W, De Muinck Keizer-Schrama SM, et al. The diagnostic work up of growth failure in secondary health care; an evaluation of consensus guidelines. BMC Pediatr. 2008;8:21.
Lashari SK, Korejo HB, Memon YM. To determine frequency of etiological factors in short statured patients presenting at an endocrine clinic of a tertiary care hospital. Pak J Med Sci. 2014;30(4):858-861.
Cheetham T, Davies JH. Investigation and management of short stature. Arch Dis Child. 2014;99(8):767-771.
Morgan T. Turner syndrome: diagnosis and management. Am Fam Physician. 2007;76(3):405-410.
Trotter TL, Hall JG American Academy of Pediatrics Committee on Genetics. Health supervision for children with achondroplasia [published correction appears in Pediatrics . 2005;116(6):1615]. Pediatrics. 2005;116(3):771-783.
Davies JH, Cheetham T. Investigation and management of tall stature. Arch Dis Child. 2014;99(8):772-777.
Kumar S. Tall stature in children: differential diagnosis and management. Int J Pediatr Endocrinol. 2013(suppl 1):P53.
Storr HL, Chan LF, Grossman AB, Savage MO. Paediatric Cushing's syndrome: epidemiology, investigation and therapeutic advances. Trends Endocrinol Metab. 2007;18(4):167-174.
Rogol AD, Hayden GF. Etiologies and early diagnosis of short stature and growth failure in children and adolescents. J Pediatr. 2014;164(5 suppl):S1-S14.e6.
Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000;85(11):3990-3993.
Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab. 2007;92(3):804-810.
Leschek EW, Rose SR, Yanovski JA, et al.; National Institute of Child Health and Human Development-Eli Lilly & Co. Growth Hormone Collaborative Group. Effect of growth hormone treatment on adult height in peripubertal children with idiopathic short stature: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(7):3140-3148.
Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database Syst Rev. 2007;3:CD004440.
Cohen P, Rogol AD, Deal CL, et al. 2007 ISS Consensus Workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210-4217.
de Waal WJ, Greyn-Fokker MH, Stijnen T, et al. Accuracy of final height prediction and effect of growth-reductive therapy in 362 constitutionally tall children. J Clin Endocrinol Metab. 1996;81(3):1206-1216.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2015 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
EDITORIAL article
Editorial: short stature: beyond growth hormone.

- 1 Department of Pediatrics, Second Faculty of Medicine, Charles University in Prague and Motol University Hospital, Prague, Czechia
- 2 Endocrinology and Diabetes Unit, “Bambino Gesù” Children’s Hospital, Rome, Italy
- 3 Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
- 4 Department of Medicine, Surgery and Health Sciences, University of Trieste, Trieste, Italy
- 5 Institute for Maternal and Child Health IRCCS “Burlo Garofolo”, Trieste, Italy
Editorial on the Research Topic Short stature: beyond growth hormone
Short stature is the most common cause of referral to pediatric endocrinology units ( 1 ) and can be defined as a multifactorial condition regulated by genetic, epigenetic, and environmental factors ( 2 ). Traditionally, growth hormone (GH) has been considered the main regulator of growth. However, as understanding of short stature pathogenesis advances, a new concept has been proposed: the role of GH in the regulation of growth is only one of many factors influencing growth plate physiology ( 3 ). Moreover, the diagnostic methods currently used to diagnose GH deficiency (GHD) are known to have low specificity, leading to frequent false positive results ( 4 ). Children with diagnosed GHD are therefore believed to have variable etiology of their growth disorder, frequently independent of GH secretion ( 5 ).
One of the major topics in current pediatric endocrinology is whether it is possible to improve the poor accuracy of GH stimulation tests. One possibility might be the optimization of sex-steroid priming ( 6 ). Partepone et al. presented a comprehensive review regarding this topic. The authors highlighted a close link between sex steroids and GH secretion leading to a higher probability of false positive results in children with delayed onset of puberty and consequent GH overtreatment. The same mechanism, on the other hand, may lead to a non-physiological GH peak, resulting in missing the diagnosis in children with real GH deficiency (GHD) in case sex-steroid priming is performed. So far, there is no agreement Regarding the indication and management of sex-steroid priming. Another issue that might lead to an inaccurate diagnosis of GHD is bone age (BA) evaluation. Delayed BA is mandatory before making the GHD diagnosis in some countries ( 7 ), however, the subjective nature of the evaluation is considered its main disadvantage. Maratova et al. evaluated an automated software for BA evaluation and proved its good accuracy.
A lasting controversy in the current way to diagnose GHD was supported by Plachy et al. Using next-generation sequencing methods, the authors genetically examined children with isolated growth hormone deficiency (GHD) and familial short stature. Interestingly, the genetic results frequently did not correspond with the previous diagnosis of GHD – 67% of children with a clinical diagnosis of GHD and a genetic etiology of short stature had proven primary growth plate disorder. Another point of view on the same topic was presented by Lanzetta et al. In their retrospective analysis of children with a clinical and laboratory diagnosis of GHD, they compared children with or without an identifiable genetic, functional, or anatomical cause of GHD, namely definite GHD or short stature unresponsive to stimulation tests (SUS). These two groups differed significantly in pretreatment IGF-1 concentration and their increase after GH treatment initiation, in prevalence of pathological retesting, and of being overweight/obese at the end of treatment. However, the response to GH treatment in terms of near-adult height did not differ between the groups. Despite lasting doubts regarding the accuracy of GHD diagnostics, children diagnosed with “GHD” might profit from GH therapy even when another etiology of short stature is suspected.
The etiology of short stature other than GHD was covered by two other articles in our Research Topic. Mastromauro et al. wrote a review presenting growth hormone insensitivity (GHI) as a broad spectrum of disorders with a variable clinical picture. Since Laron described homozygous mutations in the gene for the GH receptor as the first mechanism causing GHI, many novel causes of GHI have been described, demonstrating the complexity of GHI and its role in the growth regulation. Another numerous and etiologically highly variable group of children are those born small for gestational age (SGA) with persistent short stature ( 8 ). In a retrospective study, Becker et al. compared clinical features and responses to GH treatment of SGA children with and without syndromic signs. They discovered that syndromic SGA children were shorter at the initiation of GH treatment, started GH therapy earlier, and reached a shorter adult height despite receiving higher doses of GH.
The etiology of growth disorders is, therefore, more complex than originally expected and is not just a matter of hormones. To understand it better, we must think far beyond GH.
Author contributions
LP: Writing – original draft, Writing – review & editing. AD: Writing – original draft, Writing – review & editing. GT: Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bellotto E, Monasta L, Pellegrin MC, Bossini B, Tamaro G, Conte MS, et al. Pattern and features of pediatric endocrinology referrals: A retrospective study in a single tertiary center in Italy. Front Pediatr . (2020) 8:580588. doi: 10.3389/fped.2020.580588
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Dauber A, Rosenfeld RG, Hirschhorn JN. Genetic evaluation of short stature. J Clin Endocrinol Metab . (2014) 99:3080–92. doi: 10.1210/jc.2014-1506
3. Baron J, Savendahl L, Luca F, Dauber A, Philip M, Wit JM, et al. Short and tall stature: A new paradigm emerges. Nat Rev Endocrinol . (2018) 6:736–46. doi: 10.1038/nrendo.2015.165
CrossRef Full Text | Google Scholar
4. Bright GM, Morris PA, Rosenfeld RG. When is a positive test for pediatric growth hormone deficiency a true-positive test? Horm Res Paediatr . (2021) 94:399–405. doi: 10.1159/000521281
5. Tornese G. Growth hormone deficiency or rather “short stature unresponsive to stimulation tests”. Arch Dis Child . (2023) 108:176–7. doi: 10.1136/archdischild-2021-323426
6. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor–I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Pediatr . (2017) 86:361–79. doi: 10.1159/000452150
7. Binder G, Reinehr T, Ibanez L, Thiele S, Linglard A, Woelfle J. GHD diagnostics in europe and the US: an audit of national guidelines and practice. Horm Res Pediatr . (2019) 92:150–6. doi: 10.1159/000503783
8. Toni L, Plachy L, Dusatkova P, Amaratunga SA, Elblova L, Sumnik Z, et al. The genetic landscape of children born small for gestational age with persistent short stature. Horm Res Pediatr . (2024) 97:40–52. doi: 10.1159/000530521
Keywords: endocrinological diseases, stimulation tests, growth hormone, genetics, short stature
Citation: Plachy L, Deodati A and Tornese G (2024) Editorial: Short stature: beyond growth hormone. Front. Endocrinol. 15:1403112. doi: 10.3389/fendo.2024.1403112
Received: 18 March 2024; Accepted: 22 March 2024; Published: 28 March 2024.
Edited and Reviewed by:
Copyright © 2024 Plachy, Deodati and Tornese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianluca Tornese, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Journal Menu
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
- arrow_forward_ios Forthcoming issue arrow_forward_ios Current issue
- Vol. 13 (2024)
- Vol. 12 (2023)
- Vol. 11 (2022)
- Vol. 10 (2021)
- Vol. 9 (2020)
- Vol. 8 (2019)
- Vol. 7 (2018)
- Vol. 6 (2017)
- Vol. 5 (2016)
- Vol. 4 (2015)
- Vol. 3 (2014)
- Vol. 2 (2013)
- Vol. 1 (2012)
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
Short Stature in Children: Diagnosis, Management, and Treatment
Special issue editors, special issue information, benefits of publishing in a special issue.
- Published Papers
A special issue of Journal of Clinical Medicine (ISSN 2077-0383). This special issue belongs to the section " Clinical Pediatrics ".
Deadline for manuscript submissions: closed (25 June 2023) | Viewed by 6696
Share This Special Issue

Dear Colleagues,
Between 0.6% and 2.3% of healthy children are short. Short stature in children is the one of the most frequent reasons for consultation in a Pediatric Endocrinology Unit. Almost no patient has pathology and can be studied in primary care.
However, with the standard clinical and analytical evaluation, a diagnosis is not reached in some cases, which fall within the so-called idiopathic short stature.
Recent hormonal and genetic studies are making it possible to reduce the percentage of unknown causes and to approach the specific etiology with consequent improvement in genetic treatment and counseling.
Another prominent issue is the use of growth hormones, with indications approved (some very recently) by the EMA and FDA, although not always coincidently (such as idiopathic short stature) and with diverse inclusion criteria that invite the follow-up of these patients up to adult height and investigate predictive factors of response. Long-term follow-up is also important to ensure patients’ safety.
In this Special Issue, we aim to cover current knowledge regarding growth pathology, its endocrinological and genetic involvement, and the most appropriate therapeutic measures.
Dr. Juan Pedro López-Siguero Dr. José-Ignacio Labarta-Aizpún Guest Editors
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website . Once you are registered, click here to go to the submission form . Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Clinical Medicine is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
- short stature
- idiopathic short stature
- growth factors
- growth hormone
- Ease of navigation: Grouping papers by topic helps scholars navigate broad scope journals more efficiently.
- Greater discoverability: Special Issues support the reach and impact of scientific research. Articles in Special Issues are more discoverable and cited more frequently.
- Expansion of research network: Special Issues facilitate connections among authors, fostering scientific collaborations.
- External promotion: Articles in Special Issues are often promoted through the journal's social media, increasing their visibility.
- e-Book format: Special Issues with more than 10 articles can be published as dedicated e-books, ensuring wide and rapid dissemination.
Further information on MDPI's Special Issue polices can be found here .
Published Papers (3 papers)

Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Research article
- Open access
- Published: 28 March 2020
Final adult height of children with idiopathic short stature: a multicenter study on GH therapy alone started during peri-puberty
- Rui-min Chen 2 ,
- Shao-ke Chen 3 ,
- Ge-li Liu 4 ,
- Lin-qi Chen 5 ,
- Yu Yang 6 ,
- Xin-li Wang 7 ,
- Ya-guang Peng 8 &
- Chun-xiu Gong 1
BMC Pediatrics volume 20 , Article number: 138 ( 2020 ) Cite this article
6009 Accesses
12 Citations
6 Altmetric
Metrics details
To evaluate the efficacy of GH in improving FAH in ISS children in a multicenter study.
A real-world observation was carried out. Children with ISS in seven hospitals in China were enrolled. The height gains standard deviation score and the height gain over the target height were evaluated.
There were 344 ISS patients (217 boys and 127 girls). The baseline average age of boys and girls was 12.7 and 11.7 years, with bone age of 11.7 and 10.1 years, respectively. The baseline height SDS of boys and girls was − 3.07 and − 2.74, and the FAH SDS was − 1.91 and − 1.38, respectively. Compared with the baseline height SDS, the FAH SDS was significantly increased in both boys and girls (both P = 0.0000). The FAH SDS was the highest (gain by 1.54 SD) in the ≥2y treatment course group. Two hundred eighteen patients (218/344, 63.4%) had a FAH SDS > − 2 SD. Among these patients, girls in the 1-2y treatment course group and ≥ 2y group had a FAH SDS higher than TH SDS. Even in the control group, a spontaneous catch-up growth of 1.16 SD was observed. A multivariate linear regression model was used to analyze the results, with FAH SDS as the dependent variable. It was found that the treatment course and baseline height SDS in the boys’ model were statistically significant ( P < 0.05), whereas the baseline height SDS and baseline bone age significantly affected the girls’ FAH SDS ( P < 0.05).
Conclusions
Both girls and boys of ISS improved FAH by GH therapy even if treatments begin over 10 years old and majority of them reached TH. Some peri-puberty ISS will have a spontaneous height gain. We recommend the course of GH treatment more than 2 years for girls, and longer courses for boys.
Peer Review reports
Idiopathic short status (ISS) is defined as a condition in which the height of an individual is more than 2SD score (SDS) below the corresponding mean height for a given age, sex, and population group without evidence of systemic, endocrine, nutritional, or chromosomal abnormalities. Children with ISS have normal birth weight and are GH sufficient [ 1 ]. The incidence of ISS (including constitutional delay of growth and puberty and familial short stature) is about 23 in 1000 [ 1 , 2 , 3 ]. In 2003, the US Food and Drug Administration approved growth hormone (GH) for the treatment of ISS patients (height < − 2.25 SD). The main purpose of GH therapy for ISS is to attain normal adult height and avoid daily life inconvenience and psychological problems caused by extreme or unacceptable short stature. However, few clinical studies have explored whether the final adult height (FAH) can reach the normal range after GH therapy. FAH is considered the golden indicator for evaluating the efficacy of GH therapy [ 4 , 5 ]. However, the predicted FAH following a short period of treatment is dynamic and cannot reflect the actual FAH. Owing to the heterogeneity of the treated ISS populations and the individualization of treatment, only a few randomized trials with small sample sizes have observed ISS until FAH [ 6 , 7 , 8 , 9 , 10 ]. A randomized study of FAH typically takes 8 years or more to complete and is often difficult to implement in clinical settings. Thus, most of the currently available studies only have small samples and are carried out in a single center. More studies are needed to confirm the efficacy of GH in the treatment of ISS.
In the clinical real world, many ISS children’s parents are willing to observe their children growth when they are young. As children grow older and become peri-puberty, more people come to see doctors. What is the effect of GH treatment alone in peri-puberty? Past literatures are not accurate. This is the first multicenter study in China on the efficacy of growth hormone alone in the treatment of elder children. In our current study, we followed up children with ISS diagnosed by the departments of pediatric endocrinology in seven tertiary hospitals in different regions of mainland China, and evaluated the efficacy of GH for ISS children until FAH.
Patients with ISS confirmed in the departments of pediatric endocrinology in seven tertiary hospitals, namely Beijing Children’s Hospital Affiliated to Capital Medical University, Fuzhou Children’s Hospital of Fujian Medical University Teaching Hospital, The second Affiliated Hospital of Guangxi Medical University, General Hospital of Tianjin Medical University, Children’s Hospital of Soochow University, Children’s Hospital of Jiangxi Province, and Third Hospital of Peking University, were enrolled in this study.
The inclusion criteria included: (a) body height less than − 2 SD of the height in the general population with the same race, age, sex, and other factors; (b) without systemic disease, endocrine disease, nutritional disease, or chromosomal abnormality; (c) with normal body length and weight at birth; (d) with a serum peak GH concentration > 7 ng/mL at peak GH stimulation test and normal insulin-like growth factor 1; (e) born before January 1, 2001; (f) had been treated with GH alone for ISS but the GH therapy had been withdrawn and FAH reached, regardless of whether the patient was in Tanner stage 1 at admission; and (g) informed consent was signed by parents and children older than 8 years.
The exclusion criteria included: (a) children who were treated with GnRHa (gonadotropin-releasing hormone agonist); and (b) obese children.
Regarding the concept of peri-puberty, there is no well-recognized age limit for its definition. Since the baseline Tanner stages differed in our research populations, the term peri-puberty was used in our study.
All patients and their parents signed informed consent for data collection.
A real-world observation was carried out. Clinical data including name, sex, date of birth, age at baseline, baseline height, baseline bone age based on Greulich-Pyle methodology, GH treatment course, age at last follow-up, FAH, and parents’ heights were recorded. The baseline height SD score (SDS), FAH SDS, and target height (TH) SDS were calculated. GH was subcutaneously injected at a dose of 0.15–0.2 IU/kg/day.
The height SD score (HtSDS) was calculated by referring to the 2005 Standard Deviations of Height and Weight for Children and Adolescents Aged 0–18 years in China [ 11 ].
Height standard deviation score (HtSDS) = (actual height − average height for children of the same sex and age) ÷ (SD of the heights for children of the same sex and age) [ 11 ].
TH (i.e., mid-parental target height) was as follows:
FAH was defined as follows: age at the last follow-up was > 15 years, height velocity was below 1 cm/year, and the GH therapy had been discontinued [ 12 ].
The primary endpoint was the difference between FAH SDS and baseline height SDS, i.e., the height SDS gain, expressed as △ 1HtSDS. The secondary endpoint was the difference between FAH SDS and TH SDS, i.e., the height gain over the TH ( △ 2HtSDS). △ 3HtSDS is baseline height SDS minus TH SDS. In addition, influencing factors of height SDS were analyzed.
The control group comprised patients who had been treated for less than 3 months. The remaining patients were divided into the 3-6 m group (treated for 3–6 months), 6-12 m group (treated for 6 months to 1 year), 1-2y group (treated for 1–2 years), and ≥ 2y group (treated for 2 or more years).
Patients were also stratified according to gender.
Screening flowchart:
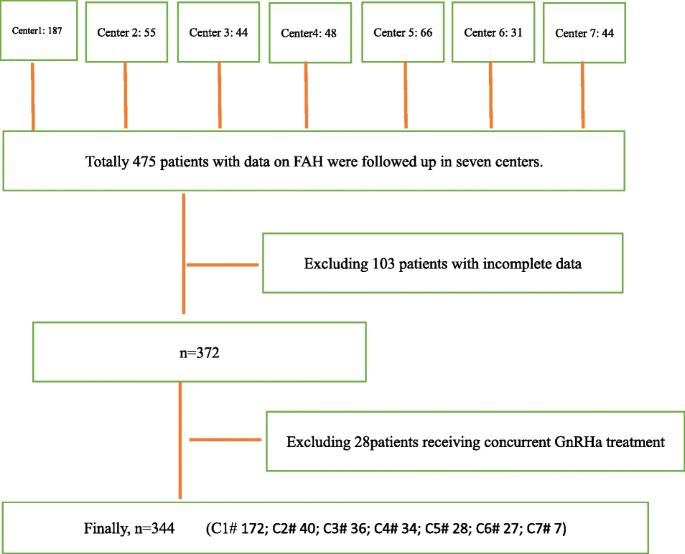
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software. A normal distribution test showed that all measurement data were normally distributed. Data are presented as mean ± SD. The means of two independent samples were compared by using the t test, and the comparisons of means among multiple groups were based on analysis of variance. The influencing factors of FAH SDS were analyzed by multivariate linear regression. A P value of less than 0.05 was considered significantly different.
General data
Among the 344 ISS patients in seven centers, there were 217 boys and 127 girls. The average age of boys and girls when starting the treatment (baseline) was 12.7 ± 1. 87 and 11.7 ± 1.61 years, with bone age of 11.7 ± 2.32 years and 10.1 ± 2.03 years, respectively. The growth stopped at the final follow-up visit, with a mean age of 18.5 ± 2.25 years for boys and 18.0 ± 2.02 years for girls (Table 1 ).
The baseline height SDS of boys and girls was − 3.07 and − 2.74, and the FAH SDS was − 1.91 and − 1.38, respectively. Compared with the baseline height SDS, the FAH SDS was significantly increased in both boys and girls (both P = 0.0000) (Table 1 ).
Comparisons between FAH SDS and baseline height SDS
According to the treatment course, patients who had been treated for less than 3 months were designated the control group, and the remaining patients were divided into the 3-6 m, 6-12 m, 1-2y, and ≥ 2y groups. The average course of treatment was 2.92 years in the ≥2y group. The baseline height SDS and TH SDS were comparable among the five groups.
The baseline ages of the control group, 3-6 m group, and 6-12 m group were significantly larger than that of the ≥2y group.
The height gain SDS (i.e., between FAH SDS and baseline height SDS [ △ 1HtSDS]) was 1.16, 1.30, 1.00, 1.01, and 1.54 in each group; compared with the control group, the P value was 0.503, 0.492, 0.525, and 0.082, showing no significant difference. However, the FAH SDS was highest (increased by 1.54 SD) in the ≥2y group. The △ 1HtSDS showed a gradually increasing trend in the 6-12 m group, 1-2y group, and ≥ 2y group. Compared with the other two groups, the ≥2y group had significantly different △ 1HtSDS ( P = 0.022). Even in the control group (regarded as an untreated group), a spontaneous catch-up growth of 1.16 SD was observed. However, the absolute height gain ( △ 1HtSDS) was clinically significantly higher in ≥2y group than in the control group (1.54 versus 1.16). In each group, there was significant difference between △ 2HtSDS and △ 3HtSDS. (Table 2 ; Fig. 1 and Supp. Figure 1 ).
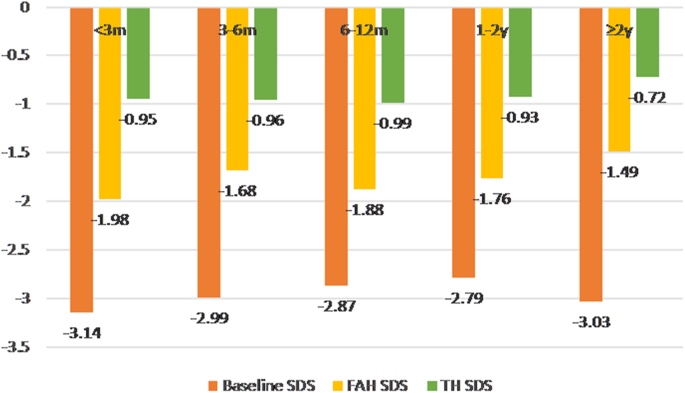
Baseline height SDS, FAH SDS, and TH SDS in each group
Comparisons of FAH SDS and TH SDS
According to the Chinese Children Growth Standards, an SDS value of 1 is considered a clinically acceptable boundary value with practical clinical significance. Based on the gender stratification, the difference between FAH SDS and TH SDS was compared. The FAH SDS (− 1.913) was 1.079 lower than the TH SDS (− 0.834) (95% confidence interval [CI]: − 1.279 to − 0.878, P > 0.05) in boys and 0.398 lower (95% CI: − 0.625 to − 0.170, P < 0.001) in girls. Thus, the FAH SDS was closer to the TH SDS in girls after treatment.
Analysis of patients with FAH attaining normal height
In total, 218 patients (218/344, 63.4%) had a FAH SDS > − 2 SD, attaining the normal and non-short height (Table 3 ), comprising 118 boys (118/217, 54.4%) and 100 girls (100/127, 78.7%). Among these patients, girls in the 1-2y group and ≥ 2y group had a FAH SDS higher than TH SDS. FAH SDS was lower than TH SDS in all groups of boys who attained the normal and non-short height (Table 3 and Supp. Figure 2 ).
Compared with the pooled group with a FAH attaining the normal height, 8 of 10 girls and 11 of 31 boys in the control group attained the normal adult height. There was statistical significance between the number of boys in the control group and that in the pooled group ( P = 0.049); that is, the number of boys in the control group attaining the normal height was smaller than that of boys in the pooled group reaching the normal height, while there was no significant difference between the control group and the pooled group.
Analysis of influencing factors of FAH
A multivariate linear regression model was used to analyze the results, with FAH SDS as the dependent variable and the baseline age, baseline bone age, baseline height SDS, treatment course, and TH as independent variables. It was found that treatment course and baseline height SDS in the boys’ model were statistically significant ( P < 0.05), whereas the baseline height SDS and baseline bone age affected the girls’ FAH SDS (Table 4 ).
This is the first observational multicenter study with FAH results in China on the efficacy of growth hormone alone for ISS. It was an observational study based on the clinical reality and reflected the real-world situation. In addition to the follow-up of FAH, the difference between FAH SDS and baseline height SDS and between FAH height SDS and TH SDS was also evaluated. It was found that a treatment course of 2 years or more had better efficacy. Even for over 10 years old ISS children, GH therapy could improve FAH if the treatment course was long enough.
Randomized controlled trials (RCTs) have been recognized as high-quality research evidence because of their rigorous grouping/screening criteria and standardized treatment. However, sometimes RCTs do not actually reflect the clinical reality because the efficacy of GH for ISS varies greatly and the GH treatment is highly individualized in the true clinical setting. Although real-world retrospective studies are feasible, their data are limited by the natural characteristics of the treated children. In the study of short stature, it is difficult to find a control group without treatment: in clinical practice, few children will adhere to the follow-up protocol if no GH treatment is applied. In the current study, patients who had received GH treatment for less than 3 months were included in the control group, which was based on the following consideration: although GH is effective for a patient, such efficacy within a short period (3 months) can be neglected for the FAH; thus, the result of a short treatment course of up to 3 months is similar to that of natural growth. Our data comprised real-world clinical data, which may be used as evidence for long-term treatment of GH for ISS.
As known, the efficacy of GH in the treatment of ISS remains controversial. In a variety of prospective/retrospective, randomized/non-randomized, and controlled/non-controlled trials, some researchers concluded that GH treatment improved ISS while others considered it ineffective. The efficacies in these studies varied dramatically [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. Some meta-analyses showed that GH-treated ISS patients might gain more FAH than untreated children, but were still shorter than the normal populations. In the meta-analysis performed by Deodati and Canfarani, three RCTs that followed the ISS patients until FAH were included, among which the average treatment course was 4.6–6.2 years [ 8 ]. In these three RCTs the treated group gained 0.79 SDS (4.7 cm) more height than the control group without GH treatment. In the largest trial, the height attainment was 0.8 SDS (5 cm) more in the treatment group than in the control group. In the study by Sotos and Tokar, the height attainment was 1.9 SDS in the treatment group and only 0.49 SDS in the untreated control group [ 3 ]. Wit et al. evaluated 239 children treated with GH, of whom only 50 were followed up until FAH. The FAH increased by 1.52–1.85 SDS compared with baseline height [ 10 ]. In our current study, the FAH of boys and girls increased by 1.16 SD and 1.36 SD compared with baseline height, which is basically consistent with results reported in the literature. Compared with the 6 m-2y group, the ≥2y group had significantly more FAH gain over the baseline height. Therefore, GH treatment for more than 2 years can achieve better efficacy in ISS patients.
Our study showed that 54.4% of boys and 78.7% of girls had a FAH SDS > −2SD after GH treatment. This result explains that GH therapy is effective even in peri-puberty children. Among the girls who had been treated with GH > 2 years, their FAH was higher than TH. This further confirmed that GH therapy could improve FAH and longer the better. For boys, FAH SDS was lower than TH SDS in all groups although they attained the normal height. It may be that boys need to improve their height longer than girls. In the GeNeSIS observation study by Pfäffle and colleagues, including United States, Germany and France data, shows that most children achieved near adult height (NAH) within the normal range (height SDS > − 2) after GH treatment [ 19 ]. The main population of our study is Chinese Han children. It shows that the same results as in Europe and America, the efficacy of growth hormone is similar.
The average age of boys and girls at baseline was 12.7 ± 1.87 years and 11.7 ± 11.7 years, with bone age of 11.7 ± 2.32 years and 10.1 ± 10.02 years, respectively. The relatively late initiation of treatment reflected the real-world situation. Multivariate linear regression model analysis showed that FAH is influenced positively by baseline height SDS in the boys and girls, whereas negatively by baseline bone age in girls, which was consistent with previous findings [ 1 , 3 , 8 , 13 , 20 ]. Many previous studies have demonstrated that the age when starting GH treatment is an important factor for its efficacy [ 1 , 2 , 6 , 10 , 20 ]. In this regard, our patients might have benefited more from GH therapy if the treatment had started earlier.
And we notice in this study, even in the control group, there is a spontaneous height gain of 1.16 SD. Economic factors need to be taken into account in the treatment of ISS. In China, growth hormone therapy for ISS is covered by families themselves. Whether to treatment or not, as well as the course of treatment, is decided by parents after weighing the family economic and height expectations.
This study had some limitations: Although it was a multi-center observation study, 344 of 475 cases entered the final analysis. It reflects “real-world” clinical practice and can be considered reliable, but which is not represented in random clinical trials. The sample is not big enough when stratification by treatment duration, if there is a larger sample in each subgroup, it will be better to validate our findings.
In summary, both girls and boys of ISS improved FAH by GH therapy even if treatments begin over 10 years old and majority of them reached TH. Some peri-puberty ISS will have a spontaneous height gain. We recommend the course of GH treatment more than 2 years for girls, and longer courses for boys.
Availability of data and materials
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
Idiopathic short status
Standard deviations
Growth hormone
Final adult height
Gonadotropin-releasing hormone agonist
Standard deviation score
Target height
Height standard deviation score
Confidence interval
Randomized controlled Trials
Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, Chernausek SD, Savage MO, Wit JM, On behalf of the 2007 ISS consensus workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the growth hormone research society, the Lawson Wilkins pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008;93(11):4210–7.
Article CAS Google Scholar
Cohen LE. Idiopathic short stature: a clinical review. JAMA. 2014;311(17):1787–96.
Article Google Scholar
Sotos JF, Tokar NJ. Growth hormone significantly increases the adult height of children with idiopathic short stature: comparison of subgroups and benefit. Int J Pediatr Endocrinol. 2014;2014(1):15.
Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, BMK B, MCS B, Cheung PT, CSY C, Cohen LE, Cohen P, Dauber A, Deal CL, Gong CX, Hasegawa Y, Hoffman AR, Hofman PL, Horikawa R, AAL J, Juul A, Kamenický P, Khadilkar V, Kopchick JJ, Kriström B, MLA L, Luo XP, Miller BS, Misra M, Netchine I, Radovick S, Ranke MB, Rogol AD, Rosenfeld RG, Saenger P, Wit JM, Woelfle J. Diagnosis, Genetics, and Therapy of Short Stature in Children: A Growth Hormone Research Society International Perspective. Horm Res Paediatr. 2019;92:1–14.
Collett-Solberg PF, Jorge AAL, Boguszewski MCS, Miller BS, Choong CSY, Cohen P, Hoffman AR, Luo XP, Radovick S, Saenger P. Growth hormone therapy in children; research and practice–a review. Growth Hormon IGF Res. 2019;29(44):20–32.
Rothenbuhler A, Linglart A, Bougneres P. A randomized pilot trial of growth hormone with anastrozole versus growth hormone alone, starting at the very end of puberty in adolescents with idiopathic short stature. Int J Pediatr Endocrinol. 2015;2015(1):4.
Kelnar CJ. Growth hormone for short children--whom should we be treating and why? J R Coll Physicians Edinb. 2012;42(1):32–3.
Deodati A, Cianfarani S. Impact of growth hormone therapy on adult height of children with idiopathic short stature: systematic review. BMJ. 2011;342:c7157.
Straetemans S, De Schepper J, Thomas M, Verlinde F, Rooman R. Bespeed. Validation of prediction models for near adult height in children with idiopathic growth hormone deficiency treated with growth hormone: a Belgian registry study. Horm Res Paediatr. 2016;86(3):161–8.
Wit JM, Rekers-Mombarg LT. Dutch growth hormone advisory G. final height gain by GH therapy in children with idiopathic short stature is dose dependent. J Clin Endocrinol Metab. 2002;87(2):604–11.
Li H, Ji CY, Zong XN, Zhang YQ. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Zhonghua er ke za zhi. 2009;47(7):487–92.
PubMed Google Scholar
Ross JL, Lee PA, Gut R, Germak J. Increased height standard deviation scores in response to growth hormone therapy to near-adult height in older children with delayed skeletal maturation: results from the ANSWER program. Int J Pediatr Endocrinol. 2015;2015(1):1.
Schena L, Meazza C, Pagani S, Paganelli V, Bozzola E, Tinelli C, et al. Efficacy of long-term growth hormone therapy in short non-growth hormone-deficient children. J Pediatr Endocrinol Metab. 2017;30(2):197–201.
Ross JL, Lee PA, Gut R, Germak J. Attaining genetic height potential: analysis of height outcomes from the ANSWER program in children treated with growth hormone over 5 years. Growth Horm IGF Res. 2015;25(6):286–93.
Rothenbuhler A, Ormieres B, Kalifa G, Bougneres P. A pilot study of growth hormone administration in boys with predicted adult short stature and near-ending growth. Growth Horm IGF Res. 2015;25(2):96–102.
Graham S, Weinman J, Auyeung V. Identifying potentially modifiable factors associated with treatment non-adherence in Paediatric growth hormone deficiency: a systematic review. Horm Res Paediatr. 2018;90(4):221–7.
Rapaport R, Lee P, Ross J, Saenger P, Ostrow V, Piccoli G. Growth hormone therapy in children born small for gestational age: results from the ANSWER program. Endocr Connect. 2018;7(10):1096–104.
Richmond E, Rogol AD. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best Pract Res Clin Endocrinol Metab. 2016;30(6):749–55.
Pfäffle R, Land C, Schönau E, Holterhus PM, Ross JL, Oliveira CP, Child CJ, Benabbad I, Jia N, Jung H, Blum WF. Growth hormone treatment for short stature in the USA, Germany and France: 15 years of surveillance in the genetics and neuroendocrinology of short-stature international study (GeNeSIS). Horm Res Paediatr. 2018;90(3):169–80.
Wit JM, Clayton PE, Rogol AD, Savage MO, Saenger PH, Cohen P. Idiopathic short stature: definition, epidemiology, and diagnostic evaluation. Growth Hormo IGF Res. 2008;18(2):89–110.
Download references

Acknowledgements
The authors thank the patients, their families and the contributing physicians for their involvement in this study.
Not Applicable.
Author information
Authors and affiliations.
Department of Endocrine and Genetics and Metabolism, Beijing Children’s Hospital, Capital Medical University, National Centre for Children’s Health, No. 56 Nanlishi Road, Xicheng District, Beijing, 100045, China
Di Wu & Chun-xiu Gong
Department of Endocrinology, Fuzhou Children’s Hospital of Fujian Medical University Teaching Hospital, No.145, 817 Middle Road, Gulou District, Fuzhou, Fuzhou, 350005, Fujian Province, China
Rui-min Chen
Department of Pediatric, The second Affiliated Hospital of Guangxi Medical University, No.166, Daxuedong Road, Nanning, Guangxi, Nanning, 530007, Guangxi, China
Shao-ke Chen
Department of Pediatric, Tianjin Medical University General Hospital, No.154, Anshan Road, Heping District, Tianjin, 300052, Tianjin, China
Depatment of Endocrinology, Children’s Hospital of Soochow University, No. 92, Zhongnan Street, Gongyeyuan District, Suzhou, Suzhou, 215025, Jiangsu, China
Lin-qi Chen
Department of Endocrinology, Children’s Hospital of Jiangxi Province, No.122, Yangming Road, Donghu District, Nanchang, Jiangxi, Nanchang, 330006, Jiangxi, China
Department of Pediatric, Peking University Third Hospital, No.49, Huayuanbei Road, Haidian District, Beijing, Beijing, 100191, Beijing, China
Xin-li Wang
Center for Clinical Epidemiology and Evidence-Based Medicine, Beijing Children’s Hospital, Capital Medical University, National Centre for Children’s Health, No.56, Nanlishi Raod, Xicheng District, Beijing, Beijing, 100045, Beijing, China
Ya-guang Peng
You can also search for this author in PubMed Google Scholar
Contributions
C.G. designed the investigation, data interpretation and revised the manuscript. D.W. performed most of the investigation, data analysis and wrote and revised the manuscript. R.C., S.C., G.L., L.C., Y.Y. and X.W. performed the investigation. Y.P. contributed to statistical analysis of data. All of the authors have read and approved the manuscript.
Corresponding author
Correspondence to Chun-xiu Gong .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee of National Center for Children’s Health & Beijing Children’s Hospital Affiliated to Capital Medical University. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All data published here are under the consent for publication. Written informed consent was obtained from all individual participants over 16 years old included in the study. A parent or guardian was on behalf of any participants under the age of 16 to write informed consent.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: supp. figure 1..
△ 1HtSDS (i.e., FAH SDS minus baseline height SDS) in each group.
Additional file 2: Supp. Figure 2.
Comparisons of FAH SDS and TH SDS among girls in the pooled group with FAH SDS > − 2 SD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wu, D., Chen, Rm., Chen, Sk. et al. Final adult height of children with idiopathic short stature: a multicenter study on GH therapy alone started during peri-puberty. BMC Pediatr 20 , 138 (2020). https://doi.org/10.1186/s12887-020-02034-8
Download citation
Received : 23 July 2019
Accepted : 13 March 2020
Published : 28 March 2020
DOI : https://doi.org/10.1186/s12887-020-02034-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Final adult height (FAH)
- Idiopathic short stature (ISS)
- Standard deviation score (SDS)
- Baseline height
- Target height (TH)
BMC Pediatrics
ISSN: 1471-2431
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 04 November 2021
Short stature is associated with low flow-mediated vasodilation in Japanese men
- Takahiro Harada 1 ,
- Masato Kajikawa 2 ,
- Tatsuya Maruhashi 3 ,
- Shinji Kishimoto 3 ,
- Takayuki Yamaji 1 ,
- Yiming Han 3 ,
- Aya Mizobuchi 3 ,
- Yu Hashimoto 1 ,
- Kenichi Yoshimura 2 ,
- Yukiko Nakano 1 ,
- Kazuaki Chayama 4 ,
- Chikara Goto 5 ,
- Farina Mohamad Yusoff 3 ,
- Ayumu Nakashima 6 &
- Yukihito Higashi 2 , 3
Hypertension Research volume 45 , pages 308–314 ( 2022 ) Cite this article
179 Accesses
5 Citations
1 Altmetric
Metrics details
A Comment to this article was published on 07 December 2021
An inverse association between height and the risk of cardiovascular disease has been reported. The objective of this study was to examine the association between height and endothelial function assessed by flow-mediated vasodilation (FMD). We evaluated cross-sectional associations of height with FMD in 7682 Japanese men. All participants were divided into four groups based on height: <155.0 cm, 155.0–164.9 cm, 165.0–174.9 cm, and ≥175.0 cm. Subjects in a lower quartile of FMD were defined as subjects having low FMD values. Univariate regression analysis revealed that height was significantly correlated with FMD ( r = 0.14, p < 0.001). FMD values were 4.6 ± 3.1% in the <155.0 cm group, 5.2 ± 3.1% in the 155.0–164.9 cm group, 5.7 ± 3.1% in the 165.0–174.9 cm group and 6.1 ± 3.2% in the ≥175.0 cm group. FMD significantly increased in relation to an increase in height. Multiple logistic regression analysis revealed that higher height groups were significantly associated with a decreased risk of low FMD value compared with the <155.0 cm group after adjustments for age, presence of hypertension, dyslipidemia, diabetes, current smoking, and brachial artery diameter. FMD was low in subjects with a short stature compared with that in subjects with tall stature. Individuals with a short stature may require intensive interventions to reduce the risk of cardiovascular events.
Clinical Trial Registration Information: URL for Clinical Trials: http://www.umin.ac.jp Registration Number for Clinical Trials: UMIN000012952.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Association between atherosclerosis and height loss among older individuals
A body shape index is associated with endothelial dysfunction in both men and women
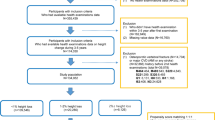
Association between height loss and cardiovascular disease in the Korean elderly
Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26.
Article CAS Google Scholar
Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–8.
Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65.
Article Google Scholar
Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF Jr, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–9.
Kajikawa M, Oda N, Kishimoto S, Maruhashi T, Iwamoto Y, Iwamoto A, et al. Increasing risk of osteoporotic fracture is associated with vascular dysfunction and abnormal vascular structure in both men and women. Circ J. 2017;81:862–9.
Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–10.
Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–72.
Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–8.
Morimoto H, Kajikawa M, Oda N, Idei N, Hirano H, Hida E, et al. Endothelial function assessed by automatic measurement of enclosed zone flow-mediated vasodilation using an oscillometric method is an independent predictor of cardiovascular events. J Am Heart Assoc. 2016;5:e004385.
Kajikawa M, Higashi Y. Promising assessment of vascular function for future cardiovascular events. J Atheroscler Thromb. 2021;28:(in press).
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128.
Hebert PR, Rich-Edwards JW, Manson JE, Ridker PM, Cook NR, O’Connor GT, et al. Height and incidence of cardiovascular disease in male physicians. Circulation. 1993;88:1437–43.
Paajanen TA, Oksala NK, Kuukasjärvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31:1802–9.
Moon J, Hwang IC. The link between height and cardiovascular disease: to be deciphered. Cardiology. 2019;143:114–5.
Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, et al. A multicenter study design to assess the clinical usefulness of semi-automatic measurement of flow-mediated vasodilatation of the brachial artery. Int Heart J. 2012;53:170–5.
American Diabetes Association Clinical practice recommendations 1999. Diabetes Care. 1999; 22 Suppl 1: S1–114.
Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–97.
Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47.
Kwok MK, Leung GM, Lam TH, Leung SS, Schooling CM. Grandparental education, parental education and child height: evidence from Hong Kong’s “Children of 1997” birth cohort. Ann Epidemiol. 2013;23:475–84.
Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–30.
Kelishadi R, Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early-life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:54–72.
Perkins JM, Subramanian SV, Davey Smith G, Özaltin E. Adult height, nutrition, and population health. Nutr Rev. 2016;74:149–65.
de Jonge LL, Harris HR, Rich-Edwards JW, Willett WC, Forman MR, Jaddoe VW, et al. Parental smoking in pregnancy and the risks of adult-onset hypertension. Hypertension. 2013;61:494–500.
Shimizu Y, Maeda T. Influence of height on endothelial maintenance activity: a narrative review. Environ Health Prev Med. 2021;26:19.
Shimizu Y, Sato S, Koyamatsu J, Yamanashi H, Nagayoshi M, Kadota K, et al. Height indicates hematopoietic capacity in elderly Japanese men. Aging. 2016;8:2407–13.
Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Brachial artery diameter as a marker for cardiovascular risk assessment: FMD-J study. Atherosclerosis. 2018;268:92–98.
O’Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O’Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–5.
Kajikawa M, Maruhashi T, Kishimoto S, Hashimoto H, Takaeko Y, Yamaji T, et al. Association of body mass index with endothelial function in Asian men. Int J Cardiol. 2021;324:186–92.
Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–13.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59.
D’Agostino RB Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7.
Download references
Acknowledgements
The authors would like to thank all of the patients who participated in this study. In addition, we thank Miki Kumiji, Megumi Wakisaka, Ki-ichiro Kawano and Satoko Michiyama for their excellent secretarial assistance; FMD-J investigators Takayuki Hidaka, MD, PhD; Shuji Nakamura, MD, PhD; Junko Soga, MD, PhD; Yuichi Fujii, MD, PhD; Naomi Idei, MD; Noritaka Fujimura, MD, PhD; Shinsuke Mikami, MD, PhD; Yumiko Iwamoto, MD; Akimichi Iwamoto, MD, PhD; Takeshi Matsumoto, MD, PhD; Nozomu Oda, MD, PhD (Department of Cardiovascular Medicine, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan); Kana Kanai, PhD; Haruka Morimoto, PhD (Department of Cardiovascular Regeneration and Medicine, Research Institute for Radiation Biology and Medicine, Hiroshima University, Hiroshima, Japan); Tomohisa Sakashita, MD, PhD; Yoshiki Kudo, MD, PhD (Department of Obstetrics and Gynecology, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan); Taijiro Sueda, MD, PhD (Department of Surgery, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan); Hirofumi Tomiyama, MD, PhD, FAHA; Akira Yamashina, MD, PhD (Department of Cardiology, Tokyo Medical University, Tokyo, Japan); Bonpei Takase, MD, PhD, FAHA (Division of Biomedical Engineering, National Defense Medical College Research Institute, Tokorozawa, Japan); Takahide Kohro, MD, PhD (Department of Cardiology, Tokyo Medical University, Tokyo, Japan); Toru Suzuki, MD, PhD (Cardiovascular Medicine, University of Leicester, Leicester, UK); Tomoko Ishizu, MD, PhD (Cardiovascular Division, Institute of Clinical Medicine, University of Tsukuba, Ibaraki, Japan); Shinichiro Ueda, MD, PhD (Department of Clinical Pharmacology and Therapeutics, University of the Ryukyu School of Medicine, Okinawa, Japan); Tsutomu Yamazaki, MD, PhD (Clinical Research Support Center, Faculty of Medicine, The University of Tokyo, Tokyo, Japan); Tomoo Furumoto, MD, PhD (Department of Cardiovascular Medicine, Hokkaido University Graduate School of Medicine, Hokkaido, Japan); Kazuomi Kario, MD, PhD (Division of Cardiovascular Medicine, Jichi Medical University School of Medicine, Tochigi, Japan); Teruo Inoue, MD, PhD (Department of Cardiovascular Medicine, Dokkyo Medical University, Mibu, Tochigi, Japan); Shinji Koba, MD, PhD (Department of Medicine, Division of Cardiology, Showa University School of Medicine, Tokyo, Japan); Kentaro Watanabe, MD, PhD (Department of Neurology, Hematology, Metabolism, Endocrinology and Diabetology (DNHMED), Yamagata University School of Medicine, Yamagata, Japan); Yasuhiko Takemoto, MD, PhD (Department of Internal Medicine and Cardiology, Osaka City University Graduate School of Medicine, Osaka, Japan); Takuzo Hano, MD, PhD (Department of Medical Education and Population-based Medicine, Postgraduate School of Medicine, Wakayama Medical University, Wakayama, Japan); Masataka Sata, MD, PhD (Department of Cardiovascular Medicine, Institute of Health Biosciences, The University of Tokushima Graduate School, Tokushima, Japan); Yutaka Ishibashi, MD, PhD (Department of General Medicine, Shimane University Faculty of Medicine, Izumo, Japan); Koichi Node, MD, PhD (Department of Cardiovascular and Renal Medicine, Saga University, Saga, Japan); Koji Maemura, MD, PhD (Department of Cardiovascular Medicine, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan); Yusuke Ohya, MD, PhD (The Third Department of Internal Medicine, University of the Ryukyus, Okinawa, Japan); Taiji Furukawa, MD, PhD (Department of Internal Medicine, Teikyo University School of Medicine, Tokyo, Japan); Hiroshi Ito, MD, PhD (Department of Cardiovascular Medicine, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Japan); and Hisao Ikeda, MD, PhD (Faculty of Fukuoka Medical Technology, Teikyo University, Omuta, Japan).
This work was supported by JSPS KAKENHI (Grant Numbers 18590815 and 21590898 to YH and JP19K17599 to MK) and a Grant in Aid of Japanese Arteriosclerosis Prevention Fund (to YH).
Author information
Authors and affiliations.
Department of Cardiovascular Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
Takahiro Harada, Takayuki Yamaji, Yu Hashimoto & Yukiko Nakano
Division of Regeneration and Medicine, Medical Center for Translational and Clinical Research, Hiroshima University Hospital, Hiroshima, Japan
Masato Kajikawa, Kenichi Yoshimura & Yukihito Higashi
Department of Cardiovascular Regeneration and Medicine, Research Institute for Radiation Biology and Medicine, Hiroshima University, Hiroshima, Japan
Tatsuya Maruhashi, Shinji Kishimoto, Yiming Han, Aya Mizobuchi, Farina Mohamad Yusoff & Yukihito Higashi
Department of Gastroenterology and Metabolism, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
Kazuaki Chayama
Department of Rehabilitation, Faculty of General Rehabilitation, Hiroshima International University, Hiroshima, Japan
Chikara Goto
Department of Stem Cell Biology and Medicine, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
Ayumu Nakashima
You can also search for this author in PubMed Google Scholar
Contributions
The study was designed by TH, MK, and YH. Data correction was conducted by TH, TY, YH, MK, CG. YH, AM, FMY, SK, TM, and AN. Data analysis and interpretation were conducted by YN, KY, and KC. Manuscript was written and approved by all authors.
Corresponding author
Correspondence to Yukihito Higashi .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Online supplement, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Harada, T., Kajikawa, M., Maruhashi, T. et al. Short stature is associated with low flow-mediated vasodilation in Japanese men. Hypertens Res 45 , 308–314 (2022). https://doi.org/10.1038/s41440-021-00785-0
Download citation
Received : 29 July 2021
Revised : 02 September 2021
Accepted : 10 September 2021
Published : 04 November 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41440-021-00785-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Short stature
- Endothelial function
- Flow-mediated vasodilation
This article is cited by
What is the connection between body height and flow-mediated vasodilation.
- Tomoaki Murakami
- Masahiro Shiraishi
Hypertension Research (2022)
Does body height affect vascular function?
Update on hypertension research in 2021.
- Masaki Mogi
- Tatsuya Maruhashi
- Kazuomi Kario
Reply to a letter to the editor regarding “Short stature is associated with low flow-mediated vasodilation in Japanese men”
- Yukihito Higashi
- Masato Kajikawa
- Takahiro Harada
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Endocrinol Metab
- v.25(5); Sep-Oct 2021
Readdressing Short Stature in India: The “Long and the Short” of it
Kv raviteja.
Department of Endocrinology, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Bhanu Malhotra
Raman k. marwaha.
1 Department of Endocrinology, International Life Sciences Institute, New Delhi, India
Pinaki Dutta
Stature is one of the widely used indices of overall health and well-being in children. Short stature (SS) can be defined either on the basis of comparison with the normal population (less than 2 SD or the 3 rd centile) or with respect to the genetic potential (derived from parental stature) of a given individual. Deviation from standard height percentiles using calculated height velocity is one of the earliest indicators of short stature, irrespective of etiology. SS evaluation is one of the most comprehensive assessments in endocrinology and entails detailed history, physical examination, and biochemical, hormonal, radiological investigations, and genetic testing when indicated. It is also the clinical situation with multiple dilemmas, bearing in mind that 25% to 90% of children referred for short stature are not actually short, reassurance is the treatment of choice in nearly half the cases, and the limited scope of the use of growth hormone (GH) therapy in idiopathic short stature (ISS), Turner syndrome (TS), and skeletal dysplasias.[ 1 , 2 ]
SS is broadly divided into normal variant short stature (NVSS) (which includes constitutional delay of growth and puberty (CDGP) and familial short stature (FSS)) or pathological short stature (causes include endocrine, syndromic, or chronic-disease related). However, FSS is increasingly being classified as a pathological variant with the recognition of hitherto undiagnosed genetic conditions such as GH resistance (GHR), collagenopathies, RASopathies, and aggrecan gene mutations.[ 3 ] RASopathies denote developmental syndromes occurring due to germline mutations in the Ras–RAF–MEK–ERK/mitogen-activated protein kinase signaling pathway and include Noonan, Leopard, and the cardio-facio-cutaneous syndromes.[ 4 ] Aggrecan is a proteoglycan constituent of the growth plate cartilage and mutations in it have been found to be the underlying cause in a proportion of patients with FSS.[ 5 ] Current understanding implicates growth plate chondrocytes as one of the central parameters of both normal growth and normal variations of growth at the intraindividual (pubertal growth spurt) and interindividual (varied normal height in the population) levels. However, there is a lack of evidence on the trends in the etiological spectrum of SS across nationwide studies. Dynamicity in the prevalence and patterns of SS is expected, considering the change in nutritional status, recognition of endocrine causes in primary care settings as well as increasing focus on identifying genetic causes of underlying growth hormone deficiency (GHD) and/or hypopituitarism. Furthermore, whether the age of identification and diagnosis of SS is decreasing, suggesting improved surveillance or awareness of this chronic problem, remains unexplored.
Studies reporting the etiology of SS in community or outpatient settings in India were analyzed and stratified on the basis of duration of study and date of publication. There were four studies in the second decade of the twenty-first century, including our unpublished survey in 2191 school children in the community,[ 6 , 7 , 8 ] and two major studies in the first decade.[ 9 , 10 ] NVSS accounted for the most number of cases of SS. Hypothyroidism was the most common endocrine cause of SS and the second most common cause overall. This evidence was consistent, irrespective of the geographical location (north and south of the country) of the participants. On the other hand, there was a discrepancy in contemporary reports from north India itself, with one study reporting skeletal dysplasia as the most common etiology of SS, followed by ISS and TS in their cohort. The variance, despite a similar time frame and similar geographic location, is intriguing but may be indicative of the settings of the study (genetic referral clinic).[ 8 ]
Nationwide trends revealed a greater preponderance of pathological SS in the first decade of the twenty-first century, which has transitioned to NVSS over the second decade. The earlier predominance of pathological SS was uniform, irrespective of consecutive admissions or referral for SS.[ 11 ] This shift may possibly be due to increased awareness and access to health care, improved trends in nutrition as well as early diagnosis and management of chronic diseases and endocrinological conditions which are implicated in SS. The overall prevalence of hypopituitarism including GHD has seemingly halved with time, from 22.6% in the twentieth century to under 10% in more recent studies, but there are/is no similar evidence available for Turner's syndrome.[ 9 , 10 ] Pathological SS is expected to be more discernible owing to greater deviations of height from the normal or clinical features associated with the primary condition (systemic or syndromic disease or hormonal cause). Hence, improved knowledge about the problem may be one of the contributors to this transition. Inherent differences between community-based and clinic-based assessments are expected owing to referral bias, as children with greater severity of SS and pathologic causes are more likely to present in hospital settings. Etiological differences may also mirror the socio-economic and literacy status of the community as malnutrition and chronic diseases are more likely to affect children from the lower strata of society. This impact of nutrition on stature is well-evidenced by the fact that the earlier reported entity of GHR, of which poor nutritional status is a cause, is virtually absent from recent studies.[ 10 , 12 ] Interestingly, the prevalence of SS has remained virtually unchanged over the past two decades in India (varying from 2.8% to 3.7%). Furthermore, the variability in growth charts between different studies has not posed a significant effect on the overall prevalence of SS. Despite this reassuring data suggesting a transition to a predominantly physiological etiology of SS, the higher age at presentation in some reports is a cause of concern.[ 7 ]
The authors performed the largest community-based study in the country to generate normative reference range intervals for IGF-1. In this study, stature was assessed among other anthropometric parameters in healthy school-going children in the community between the ages of 5 and 18 years. The final enrolment was 2191 children, with 47.92% (n = 1050) girls. We found a 2.42% (n = 53) prevalence of short stature, as defined by height < 3rd centile on the revised Indian Academy of Pediatrics (IAP) growth charts,[ 13 ] and the mean age of diagnosis of SS was 11.8 ± 4.5 years. Based on gender, the prevalence of SS was 2.54% (n = 28) in boys at a mean age of 10.5 ± 4.4 years and 2.38% (n = 25) in girls at a mean age of 13.3 ± 4.2 years. In this SS cohort, low weight (60.7%) and normal weight (39.3%) was noted in boys, but in girls, the major proportion (72%) was constituted by normal-weight individuals. Prepubertal status was present in 58.6% of the boys and 20.8% of the girls. On analyzing the causes, CDGP was present in 28% of the boys irrespective of the chart used to compute bone age (Greulich Pyle (GP) or Tanner Whitehouse (TW) charts), but the prevalence was 12% in girls when using GP charts and 16% when using TW charts. Familial short stature (FSS) was present in a higher proportion in boys (25%) than in girls with SS (20%) when using the IAP criteria and 14.2% and 8% in boys and girls, respectively, when using the National Institute of Nutrition (NIN) criteria.[ 14 ] Among pathological causes of SS, hypothyroidism was more common in girls (16%) than boys (10.7%). The overall prevalence of celiac disease in the study (defined by IgA anti-TTG) was 1.55% (n = 34), but there were no cases among children with SS. This was most likely attributable to the early pick-up/detection as they were identified in the presymptomatic phase. It may also be due to the fact that the prevalence of the coeliac disease is higher in hospital settings as children with gastrointestinal symptoms or anemia are more likely to seek consultation than in community settings where asymptomatic individuals are much more likely. Its controversial to comment so early as the IGF-1 values mismatch yet to be sorted out soon
Our study provides interesting insights into the transition in the profile and etiology of SS, which is possibly reminiscent of an upsurge in economy, education, and access to basic health care in the country. Though the prevalence of SS has remained reasonably stable, it is the metamorphosis in the etiological profile that deserves attention. The gender difference between the age of SS in the community is also intriguing, with girls getting diagnosed at a more advanced chronological age than boys, probably reflecting the social fabric in the community. Prior studies conducted in hospital settings from the north reported a relative preponderance of celiac disease, also presenting with SS as a monosymptomatic presentation. The current report of almost similar overall prevalence of celiac disease, but none of them having SS probably points towards early detection or asymptomatic seropositivity, thereby averting long-term complications such as SS in these children. Among endocrine conditions, the prevalence of hypothyroidism has remained unchanged with time. Community-based data such as ours are the true indicators of the burden of the insidious problem of SS and have furnished contemporary evidence on the prevalence and etiological spectrum of SS.
Stature being a continuous accrual and cumulative phenomenon is usually diagnosed later than sooner. One of the early clues for the diagnosis of short stature is a derailment of height velocity, which can be easily obtained from regular anthropometric evaluations in school records. Encouraging and educating schools, institutions, vaccine centers, and primary health care providers about the importance of at least annual anthropometry is another area that may aid in timely diagnosis and referral to tertiary care centers. Furthermore, in children with chronic disease of any etiology, referral to the endocrinologist for further evaluation and ruling out concurrent treatable hormone deficiency such as hypothyroidism, growth hormone deficiency or resistance, can improve final adult height. National policies to target screening for childhood stunting in identified high-risk areas can also be employed.
The study received funding from the Endocrine Society of India (ESI) and Society of Endocrine Health Care for Elderly Adolescents and Children (SEHEAC).
Conflicts of interest
There are no conflicts of interest.
R EFERRENCES

IMAGES
VIDEO
COMMENTS
This article reviews evaluation of short stature in childhood and options for management of idiopathic cases (e.g., familial short stature or constitutional delay of growth and puberty), including ...
Short Stature - StatPearls
Introduction and Background. The Growth Hormone Research Society (GRS) convened a 3-day workshop to provide an expert perspective on the diagnosis and therapy of short stature in children [].Short stature and growth deceleration are common pediatric concerns [].Although established diagnostic and management paradigms exist, recent advances in molecular technologies have greatly broadened our ...
The calculated prevalence of short stature was about 10.49% of the total population. The males 50.45% (56 cases) with mean age 13.3 ± 3.03 years were found to be affected slightly more than the females 49.55% (55 cases) with mean age 11.36 ± 3.08 years. A significant difference was seen in the age distribution between the males and females ...
Objective: We sought to obtain a better understanding of the burden of short stature using a systematic literature review. Methods: Studies of the burden of short stature, of any cause in adults and children, were searched using Embase, MEDLINE and Cochrane databases in April 2020, capturing publications from 2008 onwards. Case series and populations with adult-onset growth hormone deficiency ...
INTRODUCTION. Short stature is defined as a height that is 2 standard deviations (SD) or more below the mean height for individuals of the same sex and chronologic age in a given population. This translates to a height that is below the 2.3 rd percentile. The most common causes of short stature beyond the first year or two of life are familial ...
The Growth Hormone Research Society (GRS) convened a Workshop in March 2019 to evaluate the diagnosis and therapy of short stature in children. Forty-six international experts participated at the invitation of GRS including clinicians, basic scientists, and representatives from regulatory agencies and the pharmaceutical industry.
1. Introduction. Short stature is defined as a height more than two standard deviations (SDs) below the mean height of a reference population matched for age, sex and pubertal stage [1, 2].By this definition approximately 2.5% of the general population are considered to be of short stature, because 95% of the general population fall within two SDs of the mean of a normal distribution.
Short stature is a common indication for genetic evaluation. The differential diagnosis is broad and includes both pathologic causes of short stature and nonpathologic causes. The purpose of genetic evaluation for short stature is to provide accurate diagnosis for medical management and to provide prognosis and recurrence risk counseling for the patient and family. There is no evidence-based ...
Abstract. Short stature is a common reason for consulting a growth specialist during childhood. Normal height is a polygenic trait involving a complex interaction between hormonal, nutritional and psychosocial components. Genetic factors are becoming very important in the understanding of short stature. After exclusion of the most frequent ...
Evaluation of Short and Tall Stature in Children
The etiology of short stature other than GHD was covered by two other articles in our Research Topic. Mastromauro et al. wrote a review presenting growth hormone insensitivity (GHI) as a broad spectrum of disorders with a variable clinical picture. Since Laron described homozygous mutations in the gene for the GH receptor as the first mechanism ...
Abstract. Noonan, Turner, and Prader-Willi syndromes are classical genetic disorders that are marked by short stature. Each disorder has been recognized for several decades and is backed by extensive published literature describing its features, genetic origins, and optimal treatment strategies. These disorders are accompanied by a multitude of ...
Short stature has been associated with a higher incidence of cardiovascular disease in various populations [1,2,3,4,5,6,7,8,9,10,11,12].Several studies have reported a higher incidence rate of ...
Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website. ... Short stature is characteristic for Turner syndrome (TS) patients, and particular karyotype abnormalities of the X chromosome ...
Background To evaluate the efficacy of GH in improving FAH in ISS children in a multicenter study. Methods A real-world observation was carried out. Children with ISS in seven hospitals in China were enrolled. The height gains standard deviation score and the height gain over the target height were evaluated. Results There were 344 ISS patients (217 boys and 127 girls). The baseline average ...
Isolated short stature therefore represents the least severe end of the phenotypic spectrum of these disorders. One example is the short stature homeobox-containing ... As part of a research study, Hauer et al carried out an exome analysis on 254 families with ISS and identified candidate variants in 63 genes from 92 families (36%).
Deciphering short stature in children - PMC
SS and abnormalities in other body parts like limbs remain short. Summary. Human height is a polygenic and complex character contr ol by numerous genes, due to these many problems are relat ed to ...
Short stature is widely regarded to be a liability, but despite the importance commonly ascribed to the psychological impact of physique, there is a paucity of methodologically sound research on ...
Short stature (SS) is a common pediatric problem and it might be the first sign of underlying illness. Studies documenting the burden and etiological profile of SS are scarce from India and are mostly limited to data obtained from referral centers. Due to the lack of large-scale, community-based studies utilizing a standard protocol, the ...
FMD was low in subjects with a short stature compared with that in subjects with tall stature. Individuals with a short stature may require intensive interventions to reduce the risk of ...
Stature is one of the widely used indices of overall health and well-being in children. Short stature (SS) can be defined either on the basis of comparison with the normal population (less than 2 SD or the 3 rd centile) or with respect to the genetic potential (derived from parental stature) of a given individual. Deviation from standard height percentiles using calculated height velocity is ...