- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs


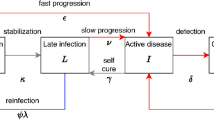
34: Tuberculosis
David Cluck
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Patient presentation.
- Full Chapter
- Supplementary Content
Chief Complaint
“I have a cough that won’t go away.”
History of Present Illness
A 63-year-old male presents to the emergency department with complaints of cough/shortness of breath which he attributes to a “nagging cold.” He states he fears this may be something worse after experiencing hemoptysis for the past 3 days. He also admits to waking up in the middle of the night “drenched in sweat” for the past few weeks. When asked, the patient denies ever having a positive PPD and was last screened “several years ago.” His chart indicates he was in the emergency department last week with similar symptoms and was diagnosed with community-acquired pneumonia and discharged with azithromycin.
Past Medical History
Hypertension, dyslipidemia, COPD, atrial fibrillation, generalized anxiety disorder
Surgical History
Appendectomy at age 18
Family History
Father passed away from a myocardial infarction 4 years ago; mother had type 2 DM and passed away from a ruptured abdominal aortic aneurysm
Social History
Retired geologist recently moved from India to live with his son who is currently in medical school in upstate New York. Smoked ½ ppd × 40 years and drinks 6 to 8 beers per day, recently admits to drinking ½ pint of vodka “every few days” since the passing of his wife 6 months ago.
Sulfa (hives); penicillin (nausea/vomiting); shellfish (itching)
Home Medications
Albuterol metered-dose-inhaler 2 puffs q4h PRN shortness of breath
Aspirin 81 mg PO daily
Atorvastatin 40 mg PO daily
Budesonide/formoterol 160 mcg/4.5 mcg 2 inhalations BID
Clonazepam 0.5 mg PO three times daily PRN anxiety
Lisinopril 20 mg PO daily
Metoprolol succinate 100 mg PO daily
Tiotropium 2 inhalations once daily
Venlafaxine 150 mg PO daily
Warfarin 7.5 mg PO daily
Physical Examination
Vital signs.
Temp 100.8°F, P 96, RR 24 breaths per minute, BP 150/84 mm Hg, pO 2 92%, Ht 5′10″, Wt 56.4 kg
Slightly disheveled male in mild-to-moderate distress
Normocephalic, atraumatic, PERRLA, EOMI, pale/dry mucous membranes and conjunctiva, poor dentition
Bronchial breath sounds in RUL
Cardiovascular
NSR, no m/r/g
Soft, non-distended, non-tender, (+) bowel sounds
Genitourinary
Get free access through your institution, pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Case report
- Open access
- Published: 19 November 2022
A case report of persistent drug-sensitive pulmonary tuberculosis after treatment completion
- Sergo A. Vashakidze 1 , 2 ,
- Abivarma Chandrakumaran 3 ,
- Merab Japaridze 1 ,
- Giorgi Gogishvili 1 ,
- Jeffrey M. Collins 4 ,
- Manana Rekhviashvili 1 &
- Russell R. Kempker 4
BMC Infectious Diseases volume 22 , Article number: 864 ( 2022 ) Cite this article
6734 Accesses
1 Citations
2 Altmetric
Metrics details
Mycobacterium tuberculosis (Mtb) has been found to persist within cavities in patients who have completed their anti-tuberculosis therapy. The clinical implications of Mtb persistence after therapy include recurrence of disease and destructive changes within the lungs. Data on residual changes in patients who completed anti-tuberculosis therapy are scarce. This case highlights the radiological and pathological changes that persist after anti-tuberculosis therapy completion and the importance of achieving sterilization of cavities in order to prevent these changes.
Case presentation
This is a case report of a 33 year old female with drug-sensitive pulmonary tuberculosis who despite successfully completing standard 6-month treatment had persistent changes in her lungs on radiological imaging. The patient underwent multiple adjunctive surgeries to resect cavitary lesions, which were culture positive for Mtb. After surgical treatment, the patient’s chest radiographies improved, symptoms subsided, and she was given a definition of cure.
Conclusions
Medical therapy alone, in the presence of severe cavitary lung lesions may not be able to achieve sterilizing cure in all cases. Cavities can not only cause reactivation but also drive inflammatory changes and subsequent lung damage leading to airflow obstruction, bronchiectasis, and fibrosis. Surgical removal of these foci of bacilli can be an effective adjunctive treatment necessary for a sterilizing cure and improved long term lung health.
Peer Review reports
Mycobacterium tuberculosis treatment has been evolving over the years, especially with the introduction of newer drugs and shorter regimens [ 1 , 2 ]. Apart from the cavitary nature of tuberculous disease, patients who have been treated with current regimens often are given the designation of cure without achieving proper sterilization. Patients who complete the tuberculous regimen are given the definition of cure after they achieve sputum negativity but many of these patients harbor bacilli within cavities that continue to exert their effects on the respiratory system [ 3 ]. The residual changes that occur in patients who have completed medical therapy have been poorly attended to in the literature. Patients that underwent surgical and medical sterilization have been reported to have better pulmonary health in the long term, especially after the removal of cavities [ 4 ].
Here, we report a patient who underwent a complete regimen of medical therapy for pulmonary tuberculosis and later had to have surgical resection of her cavities, which grew tuberculous bacilli even after achieving sputum negativity.
A 33-year-old female from the country of Georgia presented to a tuberculosis dispensary on July 10, 2020, with a temperature of 38° C and symptoms of malaise, productive cough, and night sweats. The patient had no known medical problems. She reported smoking ~ 10 cigarettes daily and denied alcohol or illicit drug use. She had 3 children and her husband was a prisoner being treated for pulmonary tuberculosis. Upon physical examination there were decreased breath sounds in the upper lobes of the lungs with dullness to percussion. The patient had a body mass index (BMI) of 16.3 kg/m 2 . A complete blood count revealed a moderate leukocytosis of 10.2 × 10 9 /L and an erythrocyte sedimentation rate (ESR) of 42 mm/h. Biochemical blood parameters were normal. Sputum testing found a negative acid-fast bacilli (AFB) microscopy, positive Xpert MTB/RIF test (no RIF resistance), and positive culture for Mycobacterium tuberculosis (Mtb). Additionally, drug susceptibility testing (DST) revealed sensitivity to rifampin, isoniazid, and ethambutol. Chest radiography revealed multiple small foci in the upper lobes of both lungs and a cavity in the right lung (Fig. 1 A). The patient was initiated on daily outpatient treatment with three pills of a fixed dosed combination pill containing isoniazid 75 mg, rifampin 150 mg, ethambutol 275 mg and pyrazinamide 400 mg. Treatment was given through directly observed therapy (DOT). She converted her sputum cultures to negative at 2 months and continued rifampin and isoniazid to finish 6 months of treatment. An end of treatment chest x-ray revealed fibrosis and honeycombing in the right upper lung, and fibrosis and dense focal shadows in the 1st and 2nd intercostal spaces of the left lung (Fig. 1 B). The complete treatment timeline is summarized in Fig. 2 .
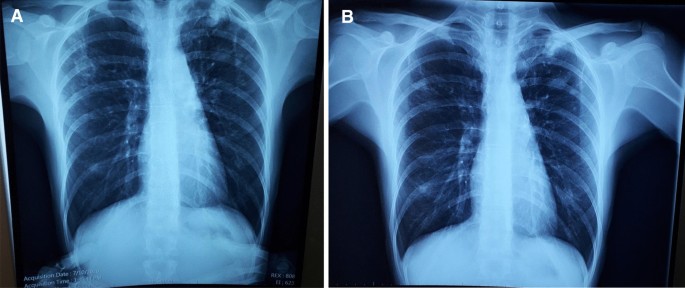
A (left): Baseline chest X-ray showing a cavity in the right lung and multiple foci in the upper lobes of both lungs. B (right): End of initial treatment chest X-ray, showing fibrosis, local honeycombing and dense focal shadows in both lungs
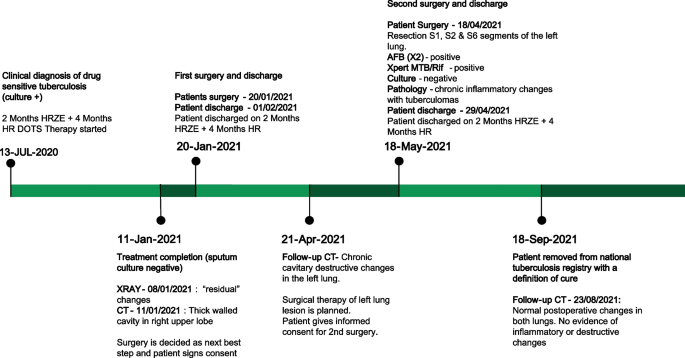
Patient treatment timeline ( HRZE isoniazid, rifampin, pyrazinamide, ethambutol; HR isoniazid & rifampin; DOTS directly observed therapy, short-course; CT computed tomography; AFB acid fast bacilli)
A follow up chest computed tomography (CT) scan demonstrated a cavity in the right upper lobe measuring 12 × 10 mm in size with a thick and heterogeneous wall and nodules and bronchiectasis in the left lung (Fig. 3 A–D). Based on CT findings and in accordance with National tuberculosis guidelines, the patient was offered surgical resection of the affected portion of the lung. It should be noted that the patient reported no symptoms, complaints, or functional disability before the surgery. Preoperative workup including pulmonary function testing, an echocardiogram, bronchoscopy, and blood chemistries were normal. The patient consented to surgery and underwent a surgical resection of the S1 and S2 segments of the right lung 2 weeks later. Intraoperatively, moderate adhesions were visualized in the S1 and S2 area with a palpable dense formation ~ 3.0 cm in diameter, in addition to a dense nodule. Gross pathology of the resected lesion showed a thick-walled fibrous cavity filled with caseous necrosis (Fig. 4 A) corresponding to the right preoperative CT lesion seen on Fig. 3 A, C.
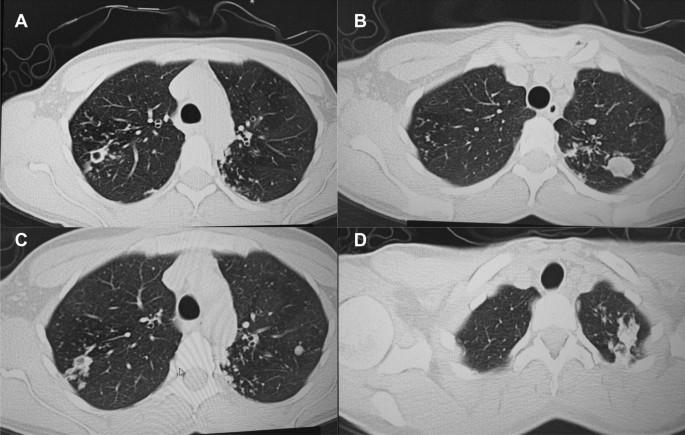
CT scan (January 11, 2021) showing, A a cavity in the upper lobe of the right lung with heterogeneous thick walls. B S1 and S2 segments of the left lung shows a 23 × 18 mm oval shaped calcified inclusions; C , D areas with calcified, compacted nodules 13 × 20 mm in size with additional traction bronchiectasis
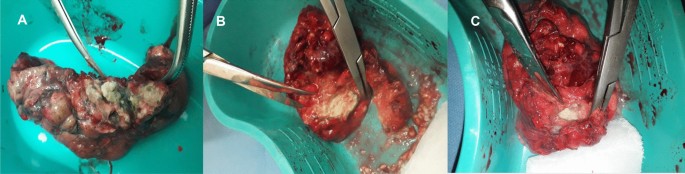
A Gross pathological image of a resected cavity with caseous material from first surgery (S1 & S2 segment of right lung). B The gross pathology from the second surgery showed the presence of a blocked cavity measuring up to 2 cm in diameter filled with caseous material in the S1, S2 and C Tuberculoma in S6 segment
Microbiological analysis on the resected tissue revealed acid-fast bacilli on microscopy, and positive Xpert MTB/RIF and culture results. Mtb grew from the caseous center, inner and outer walls of the cavity and a resected foci located ~ 3 cm from the cavity. DST revealed sensitivity to isoniazid, rifampin, and ethambutol.
Pathological examination of the resected lesion showed findings consistent with fibrocavernous tuberculosis. No postoperative complications were experienced, and the patient reinitiated first-line therapy via DOT on the 2nd postoperative day and was discharged on postoperative day 11.
A follow up CT scan performed after 3 months showed postoperative changes in the right upper lobe, and an unchanged left lung (Fig. 5 A–C). Based on the persistent conglomerate of tuberculomas and multiple small tuberculous foci, growth of Mtb from the previous surgical specimen, and the patient’s social situation (mother of three young children) a second surgery to optimize the chance of cure was recommended. The patient reported no symptoms, complaints, or functional disability before the surgery. Preoperative sputum testing found negative AFB smear microscopy and culture. The patient underwent the second operation on May 18, 2021, in which the S1, S2 and part of the S6 segment of the left lung were resected. Intraoperatively, moderate adhesions seen along with a dense palpable ~ 3 cm mass in the S1 and S2 region and a dense focus in S6.
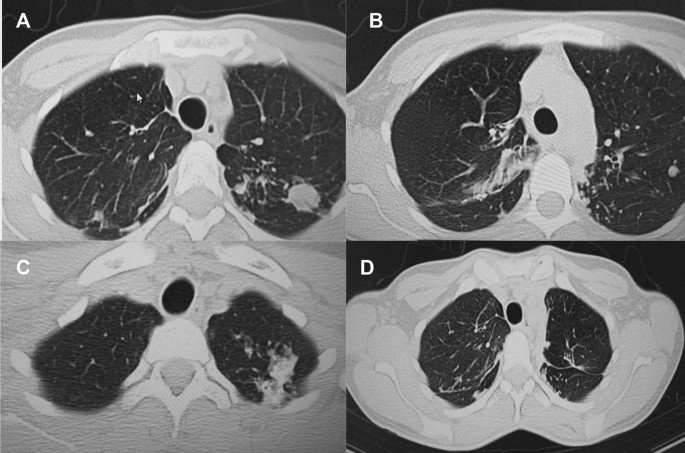
A – C Follow-up CT scan after first adjunctive surgery showing postoperative changes of the right lung and radiological changes in the left lung, that were unchanged compared to the initial CT. D Final CT scan showing normal postoperative changes with no cavities as previously seen
Microbiological examinations performed on resected tissue revealed positive AFB smear microscopy and Xpert MTB/RIF results and a negative AFB culture. The pathological examination of the surgical samples indicated a variety of destructive changes in addition to ongoing inflammation. The gross specimen of S1 and S2 segments of the left lung showed fibrocavernous tuberculosis shown in Fig. 4 B, which corresponds to the left lung lesion seen on the first preoperative CT in Figs. 3 B and 5 A in the second preoperative CT; the gross specimen of the S6 segment showed progressive tuberculoma seen in Fig. 4 C, which corresponds to the left lung lesion seen on the first preoperative CT in Figs. 3 D and 5 C in the second preoperative CT.
There were no postoperative complications, and tuberculosis (TB) treatment was reinitiated. The patient successfully completed treatment with normalization of clinical and laboratory parameters and a clinical outcome of cure in September 2021, ~ 14 months after beginning treatment. The patient had reported near complete resolution of her symptoms, having a much better ability to perform her daily activities. The patient appreciated the effects surgery had on her recovery and was happy to have gone through that treatment route. A post treatment CT scan demonstrated postoperative changes in the upper segments of both lungs (Fig. 5 D). Results from post treatment lung function testing were all within normal range.
Discussion and conclusions
We present this case to highlight the heterogeneous nature of pulmonary tuberculosis and need for an individualized treatment approach, especially for patients with cavitary disease. Over the last decade, novel diagnostics, drugs, and treatment regimens have revolutionized TB management including a recent landmark clinical trial demonstrating an effective 4-month regimen for drug-susceptible TB [ 1 ]. The move towards shorter regimens is critical to improve treatment completion rates and help meet TB elimination goals. However, during a transition to shorter treatment durations it is imperative that clinicians remain aware of complex and severe pulmonary TB cases that may require longer durations of treatment and adjunctive therapies such as surgery. Supporting evidence comes from a recent landmark study finding persistent inflammation on imaging associated with finding Mtb mRNA in sputum after successful treatment and a meta-analysis demonstrating a hard-to-treat TB phenotype not cured with the standard 6 months of treatment [ 2 , 5 ]. However, regarding recommendations for prolonging treatment beyond 6 months for drug-susceptible pulmonary tuberculosis, ATS/CDC/IDSA recommends (expert opinion) extended treatment for persons with cavitary disease and a positive 2 month culture (our patient would not have met this criteria); World Health Organization (WHO) does not recommend extended treatment for any persons with drug-susceptible TB [ 6 , 7 ]. Accumulating evidence demonstrates surgical resection may be an effective adjunctive treatment in cases with cavitary disease [ 8 , 9 , 10 , 11 , 12 ]. Ultimately, a precision medicine approach towards TB will be able to identify patients who would benefit from short course therapy and those who would benefit from longer therapy and adjunctive treatment including surgery [ 13 ].
Mtb has a unique ability and propensity to induce cavities in humans with various studies showing cavitary lesions in ~ 30 to 85% of patients with pulmonary tuberculosis [ 14 ]. Lung cavities are more common in certain groups including patients with diabetes mellitus and undernutrition such as our patient who had a baseline BMI of 16.3 kg/m 2 [ 15 , 16 ]. Their presence indicates more advanced and severe pulmonary disease as evidenced by their association with worse clinical outcomes. Cavitary disease has been associated with higher rates of treatment failure, disease relapse, acquired drug resistance, and long term-term pulmonary morbidity [ 2 , 17 , 18 , 19 ]. The impact of cavitary disease may be more pronounced in drug-resistant disease as shown in an observational study from our group which found a five times higher rate of acquired drug resistance and eight times higher rate of treatment failure among patients multidrug- or extensively drug-resistant cavitary disease compared to those without [ 20 ].
Mtb cavities are characterized by a fibrotic surface with variable vascularization, a lymphocytic cuff at the periphery followed by a cellular layer consisting of primarily macrophages and a necrotic center with foamy apoptotic macrophages and high concentrations of bacteria. Historically, each portion of the TB cavity has been conceptualized as concentric layers of a spherical structure due to its appearance on histologic cross-sections. However, recent studies using more detailed imaging techniques have shown most TB cavities exhibit complex structures with diverse, branching morphologies [ 21 ]. A dysregulated host immune response to Mtb is thought to contribute to the development of lung cavities, which may explain why cavitary lesions are seen less frequently among immunosuppressed patients including people living with Human Immunodeficiency Virus (HIV) [ 14 ]. The center of the TB cavity (caseum) is characterized by accumulation of pro-inflammatory lipid signaling molecules (eicosanoids) and reactive oxygen species, which result in ongoing tissue destruction, but do little to control Mtb replication [ 22 ]. Conversely, the cellular rim and lymphocytic cuff are characterized by a lower abundance of pro-inflammatory lipids and increases in immunosuppressive signals including elevated expression of TGF-beta and indoleamine-2,3-dioxygenase-1 [ 22 ]. The anti-inflammatory milieu within these TB cavity microenvironments impairs effector T cell responses, further limiting control of bacterial replication [ 23 , 24 , 25 ].
The combination of impaired cell-mediated immune responses with accumulation of inflammatory mediators at the rim of the caseum leads to ongoing tissue destruction with the potential for long-term pulmonary sequelae. Many with cavitary tuberculosis suffer chronic obstructive pulmonary disease after successful treatment and the risk may be greater in those with multidrug-resistant disease [ 3 , 4 ]. This has led to research into adjunctive treatment with immune modulator therapies with a goal of mitigating the over-exuberant inflammatory response at the interior edge of the cavity to limit tissue damage. In a recent randomized clinical trial, patients with radiographically severe pulmonary tuberculosis treated with adjunctive everolimus or CC-11050 (phosphodiesterase inhibitor with anti-inflammatory properties) achieved better long-term pulmonary outcomes versus those who received placebo [ 26 ]. Such results suggest the inflammatory response can be modified with appropriate host-directed therapies to improve pulmonary outcomes, particularly in those with cavitary tuberculosis.
Tuberculosis cavities not only hinder an effective immune response, but also prevent anti-tuberculosis drugs from achieving sterilizing concentrations throughout the lesion and especially in necrotic regions. The necrotic center of cavitary lesions is associated with extremely high rates of bacilli (up to 10 9 per milliliter), many of which enter a dormant state with reduced metabolic activity. Bacilli in this dormant state may be less responsive to the host immune response and exhibit phenotypic resistance to some anti-tuberculosis drugs thereby preventing sterilization and increasing chances of relapse [ 14 , 27 , 28 ]. The fact that the specimens from our patient’s second surgery were Xpert and AFB positive, but culture negative may indicate the presence of either dead bacilli or metabolically altered(dormant) bacilli that may be alive, but not culturable by standard techniques. Further, genomic sequencing studies have also found distinct strains of Mtb within different areas of the cavity that have varying drug-susceptibilities demonstrating cavities as a potential incubator for drug resistance [ 27 , 29 ].
Emerging literature has started to elucidate the varying abilities of drugs to penetrate into cavitary lesions and the importance of adequate target site concentrations. One notable study found that decreasing tissue concentrations within resected cavitary TB lesions were associated with increasing drug phenotypic MIC values [ 30 ]. Innovative studies using MALDI mass spectrometry imaging have further demonstrated varied spatiotemporal penetration of anti-TB drugs in human TB cavities [ 31 ]. This study found rifampin accumulated within caseum, moxifloxacin preferentially at the cellular rim, and pyrazinamide throughout the lesion, demonstrating the need to consider drug penetration when designing drug regimens in patients with cavitary TB. Computational modeling studies have further demonstrated the importance of complete lesion drug coverage to ensure relapse-free cure [ 32 ]. Furthermore, clinical trials are now incorporating these principles into study design by (1) using radiological characteristics to determine treatment length and (2) incorporating tissue penetration into drug selection and regimen design [ 33 , 34 ]. Beyond tissue penetration, varying drug levels and rapid INH acetylation status can also lead to suboptimal pharmacokinetics and poor clinical outcomes [ 35 , 36 ]. As highlighted in a recent expert document, clinical standards to optimize and individualize dosing need to be developed to improve outcomes [ 37 ].
Available literature points to a benefit of adjunctive surgical resection particularly among patients with drug resistant tuberculosis. A meta-analysis of 24 comparative studies found surgical intervention was associated with favorable treatment outcomes among patients with drug-resistant TB (odds ratio 2.24, 95% CI 1.68–2.97) [ 38 ]. Additionally, an individual patient data meta-analysis found that partial lung resection (adjusted OR 3.9, 95% CI 1.5–5.9) but not pneumectomy was associated with treatment success [ 39 ]. In two observational studies, we have also found that adjunctive surgical resection was associated with high and improved outcomes compared to patients with cavitary disease not undergoing surgery and was associated with less reentry into TB care. It should be noted that all studies of surgical resection for pulmonary TB were observational studies, which may be subject to selection bias, and no clinical trials (very difficult to implement in practice) were conducted to provide more conclusive evidence. Based on available evidence, the WHO has provided guidance to consider surgery among certain hard to treat cases of both drug-susceptible and resistant cavitary disease [ 40 ]. Criteria for surgical intervention included (1) failure of medical therapy (persistent sputum culture positive for M. tuberculosis ), (2) a high likelihood of treatment failure or disease relapse, (3) complications from the disease, (4) localized cavitary lesion, and (5) sufficient pulmonary function to tolerate surgery. For our patient, the severity of disease, lack of improvement of radiological imaging despite appropriate treatment, and high risk of relapse were the main indicators for surgery. Contraindications for surgery included a forced expiratory volume (FEV1) < 1000 mL, severe malnutrition, or patients at high risk for perioperative cardiovascular complications. With strict adherence to indications and contraindications for surgery, an acceptable level of postoperative complications are noted (5–17%) [ 4 , 38 ]. Our results also demonstrate the safety of adjunctive surgery, as our post-operative complication rate (8%) was low with the majority being minor complications [ 41 ].
As our case highlights, patients with persistent cavitary disease at the end of treatment require close clinical follow up and a tailored, individualized plan to determine the best approach for disease elimination and cure. In certain cases, including those with persistent cavitary disease and end of treatment, and where available, surgical resection is an effective adjunctive treatment option that can reduce disease burden and aid anti-tuberculosis agents in providing a sterilizing cure. As we enter an era of welcomed new shorter treatment options for tuberculosis it is imperative for clinicians to be able to identify and recognize complicated TB cases that require prolonged treatment and potentially adjunctive surgery.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
Acid fast bacilli
American Thoracic Society
Body mass index
Center for Disease Control
Computed tomography
Directly observed therapy
Drug sensitive tuberculosis
Erythrocyte sedimentation rate
Human Immunodeficiency Virus
Infectious Diseases Society of America
Mycobacterium tuberculosis
- Tuberculosis
World Health Organization
Dorman S, Nahid P, Kurbatova E, Phillips P, Bryant K, Dooley K, et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. 2021;384(18):1705–18.
Article PubMed PubMed Central CAS Google Scholar
Imperial M, Nahid P, Phillips P, Davies G, Fielding K, Hanna D, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med. 2018;24(11):1708–15.
Vashakidze S, Kempker J, Jakobia N, Gogishvili S, Nikolaishvili K, Goginashvili L, et al. Pulmonary function and respiratory health after successful treatment of drug-resistant tuberculosis. Int J Infect Dis. 2019;82:66–72.
Article PubMed PubMed Central Google Scholar
Harris RC, Khan MS, Martin LJ, et al. The effect of surgery on the outcome of treatment for multidrug-resistant tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:262.
Malherbe S, Shenai S, Ronacher K, Loxton A, Dolganov G, Kriel M, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med. 2016;22(10):1094–100.
Nahid P, Dorman S, Alipanah N, Barry P, Brozek J, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–95.
World Health Organization. The WHO consolidated guidelines on tuberculosis, module 4: treatment—drug-susceptible tuberculosis treatment. Geneva: WHO; 2022.
Google Scholar
Kang M, Kim H, Choi Y, Kim K, Shim Y, Koh W, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg. 2010;89(5):1597–602.
Article PubMed Google Scholar
Pomerantz B, Cleveland J, Olson H, Pomerantz M. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg. 2001;121(3):448–53.
Article PubMed CAS Google Scholar
Somocurcio J, Sotomayor A, Shin S, Portilla S, Valcarcel M, Guerra D, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax. 2007;62(5):416–21.
Dravniece G, Cain K, Holtz T, Riekstina V, Leimane V, Zaleskis R. Adjunctive resectional lung surgery for extensively drug-resistant tuberculosis. Eur Respir J. 2009;34(1):180–3.
Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg. 2008;86(5):1640–5.
Lange C, Aarnoutse R, Chesov D, van Crevel R, Gillespie S, Grobbel H, et al. Perspective for precision medicine for tuberculosis. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.566608 .
Urbanowski M, Ordonez A, Ruiz-Bedoya C, Jain S, Bishai W. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis. 2020;20(6):e117–28.
Zafar M, Chen L, Xiaofeng Y, Gao F. Impact of diabetes mellitus on radiological presentation of pulmonary tuberculosis in otherwise non-immunocompromised patients: a systematic review. Curr Med Imaging Rev. 2019;15(6):543–54.
Sinha P, Bhargava A, Carwile M, Cintron C, Cegielski J, Lönnroth K, et al. Undernutrition can no longer be an afterthought for global efforts to eliminate TB. Int J Tuberc Lung Dis. 2022;26(6):477–80.
Cegielski J, Dalton T, Yagui M, Wattanaamornkiet W, Volchenkov G, Via L, et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014;59(8):1049–63.
Gao J, Ma Y, Du J, Zhu G, Tan S, Fu Y, et al. Later emergence of acquired drug resistance and its effect on treatment outcome in patients treated with Standard Short-Course Chemotherapy for tuberculosis. BMC Pulm Med. 2016. https://doi.org/10.1186/s12890-016-0187-3 .
Shin S, Keshavjee S, Gelmanova I, Atwood S, Franke M, Mishustin S, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med. 2010;182(3):426–32.
Kempker R, Kipiani M, Mirtskhulava V, Tukvadze N, Magee M, Blumberg H. Acquired drug resistance in Mycobacterium tuberculosis and poor outcomes among patients with multidrug-resistant tuberculosis. Emerg Infect Dis. 2015;21(6):992–1001.
Wells G, Glasgow J, Nargan K, Lumamba K, Madansein R, Maharaj K, et al. Micro-computed tomography analysis of the human tuberculous lung reveals remarkable heterogeneity in three-dimensional granuloma morphology. Am J Respir Crit Care Med. 2021;204(5):583–95.
Marakalala M, Raju R, Sharma K, Zhang Y, Eugenin E, Prideaux B, et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat Med. 2016;22(5):531–8.
McCaffrey E, Donato M, Keren L, Chen Z, Delmastro A, Fitzpatrick M, et al. The immunoregulatory landscape of human tuberculosis granulomas. Nat Immunol. 2022;23(2):318–29.
Gern B, Adams K, Plumlee C, Stoltzfus C, Shehata L, Moguche A, et al. TGFβ restricts expansion, survival, and function of T cells within the tuberculous granuloma. Cell Host Microbe. 2021;29(4):594-606.e6.
Gautam U, Foreman T, Bucsan A, Veatch A, Alvarez X, Adekambi T, et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2017. https://doi.org/10.1073/pnas.1711373114 .
Wallis R, Ginindza S, Beattie T, Arjun N, Likoti M, Edward V, et al. Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir Med. 2021;9(8):897–908.
Kaplan G, Post F, Moreira A, Wainwright H, Kreiswirth B, Tanverdi M, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71(12):7099–108.
Fattorini L, Piccaro G, Mustazzolu A, Giannoni F. Targeting dormant bacilli to fight tuberculosis. Mediterr J Hematol Infect Dis. 2013;5(1):e2013072.
Moreno-Molina M, Shubladze N, Khurtsilava I, Avaliani Z, Bablishvili N, Torres-Puente M, et al. Genomic analyses of Mycobacterium tuberculosis from human lung resections reveal a high frequency of polyclonal infections. Nat Commun. 2021;12(1):2716.
Dheda K, Lenders L, Magombedze G, Srivastava S, Raj P, Arning E, et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am J Respir Crit Care Med. 2018;198(9):1208–19.
Prideaux B, Via L, Zimmerman M, Eum S, Sarathy J, O’Brien P, et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 2015;21(10):1223–7.
Strydom N, Gupta S, Fox W, Via L, Bang H, Lee M, et al. Tuberculosis drugs’ distribution and emergence of resistance in patient’s lung lesions: a mechanistic model and tool for regimen and dose optimization. PLoS Med. 2019;16(4): e1002773.
Chen R, Via L, Dodd L, Walzl G, Malherbe S, Loxton A, et al. Using biomarkers to predict TB treatment duration (predict TB): a prospective, randomized, noninferiority, treatment shortening clinical trial. Gates Open Res. 2017;1:9.
Bartelink I, Zhang N, Keizer R, Strydom N, Converse P, Dooley K, et al. New paradigm for translational modeling to predict long-term tuberculosis treatment response. Clin Transl Sci. 2017;10(5):366–79.
Pasipanodya J, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55(2):169–77.
Colangeli R, Jedrey H, Kim S, Connell R, Ma S, Chippada Venkata U, et al. Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med. 2018;379(9):823–33.
Alffenaar J, Stocker S, Forsman L, Garcia-Prats A, Heysell S, Aarnoutse R, et al. Clinical standards for the dosing and management of TB drugs. Int J Tuberc Lung Dis. 2022;26(6):483–99.
Marrone M, Venkataramanan V, Goodman M, Hill A, Jereb J, Mase S. Surgical interventions for drug-resistant tuberculosis: a systematic review and meta-analysis [review article]. Int J Tuberc Lung Dis. 2013;17(1):6–16.
Fox G, Mitnick C, Benedetti A, Chan E, Becerra M, Chiang C, et al. Surgery as an adjunctive treatment for multidrug-resistant tuberculosis: an individual patient data metaanalysis. Clin Infect Dis. 2016;62(7):887–95.
Word Health Organization Europe. The role of surgery in the treatment of pulmonary TB and multidrug and extensively drug-resistant TB. Copenhagen: WHO Regional Office for Europe; 2014.
Vashakidze SA, Gogishvili SG, Nikolaishvili KG, et al. Adjunctive surgery versus medical treatment among patients with cavitary multidrug-resistant tuberculosis. Eur J Cardiothorac Surg. 2021;60(6):1279–85. https://doi.org/10.1093/ejcts/ezab337 .
Download references
Acknowledgements
The authors thank the physicians, nurses, and staff at the NCTLD in Tbilisi, Georgia, who provided care for the patient described in this report. Additionally, the authors are thankful for the patient with pulmonary tuberculosis who was willing to have their course of illness presented and help contribute meaningful data that may help future patients with the same illness.
This study did not receive any specific funding.
Author information
Authors and affiliations.
Thoracic Surgery Department, National Center for Tuberculosis and Lung Diseases, 50 Maruashvili, 0101, Tbilisi, Georgia
Sergo A. Vashakidze, Merab Japaridze, Giorgi Gogishvili & Manana Rekhviashvili
The University of Georgia, Tbilisi, Georgia
Sergo A. Vashakidze
Tbilisi State Medical University, Tbilisi, Georgia
Abivarma Chandrakumaran
Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
Jeffrey M. Collins & Russell R. Kempker
You can also search for this author in PubMed Google Scholar
Contributions
SAV: Conceptualization; Data collection and interpretation; Scientific Writing including initial draft preparation and manuscript revision and editing. AC: Data interpretation; Table and Figure preparation; Literature review; Scientific Writing including initial draft preparation and manuscript revision and editing. MJ: Data collection; Scientific Writing including manuscript review and editing. GG: Data collection; Scientific Writing including manuscript review and editing. JMC: Data interpretation; Scientific Writing including manuscript review and editing. MR: Data interpretation; Scientific Writing including manuscript review and editing. RRK: Conceptualization; Literature review; Scientific Writing including manuscript review and editing. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sergo A. Vashakidze .
Ethics declarations
Ethics approval and consent to participate.
The authors confirm that written informed consent has been obtained from the patient involved in the case report. Ethics approval is not needed for case reports according to our institutional (National Center for Tuberculosis and Lung Disease) review board.
Consent for publication
The authors confirm that written consent has been obtained from the patient for publication of images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Vashakidze, S.A., Chandrakumaran, A., Japaridze, M. et al. A case report of persistent drug-sensitive pulmonary tuberculosis after treatment completion. BMC Infect Dis 22 , 864 (2022). https://doi.org/10.1186/s12879-022-07836-y
Download citation
Received : 08 August 2022
Accepted : 02 November 2022
Published : 19 November 2022
DOI : https://doi.org/10.1186/s12879-022-07836-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Thoracic surgery
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
CASE STUDY ON PULMONARY TUBERCULOSIS
- January 2017
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Tamil Nadu Dr. M.G.R. Medical University

- Vel's Group of Institutions
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- J T Crawford
- T M Shinnick
- JL Kamerbeek

- EMERG INFECT DIS
- Gaby E. Pfyffer

- Dick van Soolingen
- Mamadou Daffé
- Michael R. McNeil
- Patrick J. Brennan
- D Soolingen
- T Hoogenboezem
- Pwm Hermans
- K S Teppema
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
- Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal

Section navigation
- Featured topics
- Case-based TB surveillance
- Country success stories
- COVID-19, TB and India
- TB and diabetes
- TB guidelines
Digital, case-based, real-time surveillance for TB: status of progress
Tuberculosis (TB) surveillance is the continuous and systematic collection, analysis and reporting of data related to TB infection and TB disease in the population. To support countries to implement national surveillance systems for TB in a consistent and comparable way worldwide, the World Health Organization (WHO) has, since the mid-1990s, provided guidance with standardized definitions, forms, registers and reports ( 1 ). There were major updates to this guidance in 2006 ( 2 ) and 2013 ( 3 ).
A new edition of guidance on TB surveillance is in development and will be published in 2022. It will have an expanded scope that covers the full pathway of screening, diagnosis, treatment and care for people with TB infection and TB disease. It also aims to facilitate implementation of digital, case-based, real-time surveillance systems for TB, including the strengthening of systems that already exist and the transition to such systems elsewhere, especially in countries that are using a mixture of paper-based and digital systems or that rely primarily on paper-based systems.
Digital and case-based real-time surveillance systems for TB have several advantages over more traditional paper-based reporting of aggregated data. These include enabling the use of automated data quality checks, timely access to data and the availability of individual-level data for people with TB infection or disease, from the level of health facilities up to national level. These systems also greatly facilitate data analysis (including by age, sex and location) to inform adaptation and targeting of response efforts, both geographically and for specific population groups.
As of August 2021, data on the type of TB surveillance system in place at national level were available for 210 countries and territories ( Fig. 1 ). Of these, 130 reported having in place a digital, case-based surveillance system that covered all people diagnosed and reported with TB (both those with drug-susceptible TB and those with drug-resistant TB [DR-TB]). A further 14 countries, mainly in the WHO regions of Africa, the Americas and South-East Asia, had a case-based surveillance system only for people with DR-TB. Twenty countries reported that they were in the process of transitioning from a paper to digital system. About half of the countries in the WHO African Region still have paper-based systems for the recording and reporting of data.
The WHO Global TB Programme has been working with other WHO departments, the University of Oslo and the Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund) to develop and support country implementation of digital packages for the collection, analysis, visualization and use of data from routine health facility information systems ( 4 ). This has built on WHO guidance about case-based digital TB surveillance ( 5 ), guidance on the routine analysis and use of TB data ( 6 ) and the WHO TB surveillance checklist of standards and benchmarks ( 7 ). The packages are based on WHO data standards and have been developed using DHIS2 software (because many countries have already chosen DHIS2 for use within their health information systems) but can be adapted for use with other software. Each package contains a machine-readable DHIS2 configuration, an analysis guide with a core set of indicators and dashboards, and an accompanying exercise book.
A TB-specific package for the digital management, analysis and use of key surveillance data in aggregated format has been available since early 2019 ( 8 ), 1 for use by countries that are not yet ready to transition to case-based digital surveillance. The TB package for case-based data, which enables the digital management of data for both drug-susceptible TB and DR-TB in a single system, has been available since late 2020 for download as a digital data configuration package in both English and French ( 8 ). Both TB packages are based on the latest WHO recording and reporting framework, and both allow extensive data analysis at different levels of the health system (e.g. health facility and subnational administrative area). The standard dashboards include graphs, tables and maps for core surveillance indicators (e.g. notifications, coverage of testing for drug resistance and HIV, and treatment outcomes) and data quality indicators (e.g. completeness and internal consistency).
The status of implementation and use of the WHO digital package for aggregated TB data is shown in Fig. 2 . Historical subnational TB data from 60 countries have been stored and can be analysed and visualized in this package. At national level, the digital package for aggregated data has already been implemented for ongoing collection, analysis, visualization (using standard dashboards) and reporting of data in 18 countries; an additional 12 countries are in the process of doing the same. In a further 22 countries, the package has been used to upload historical data for analysis during a national TB epidemiological review. As of August 2021, piloting of the TB digital package for case-based data was underway in four countries.
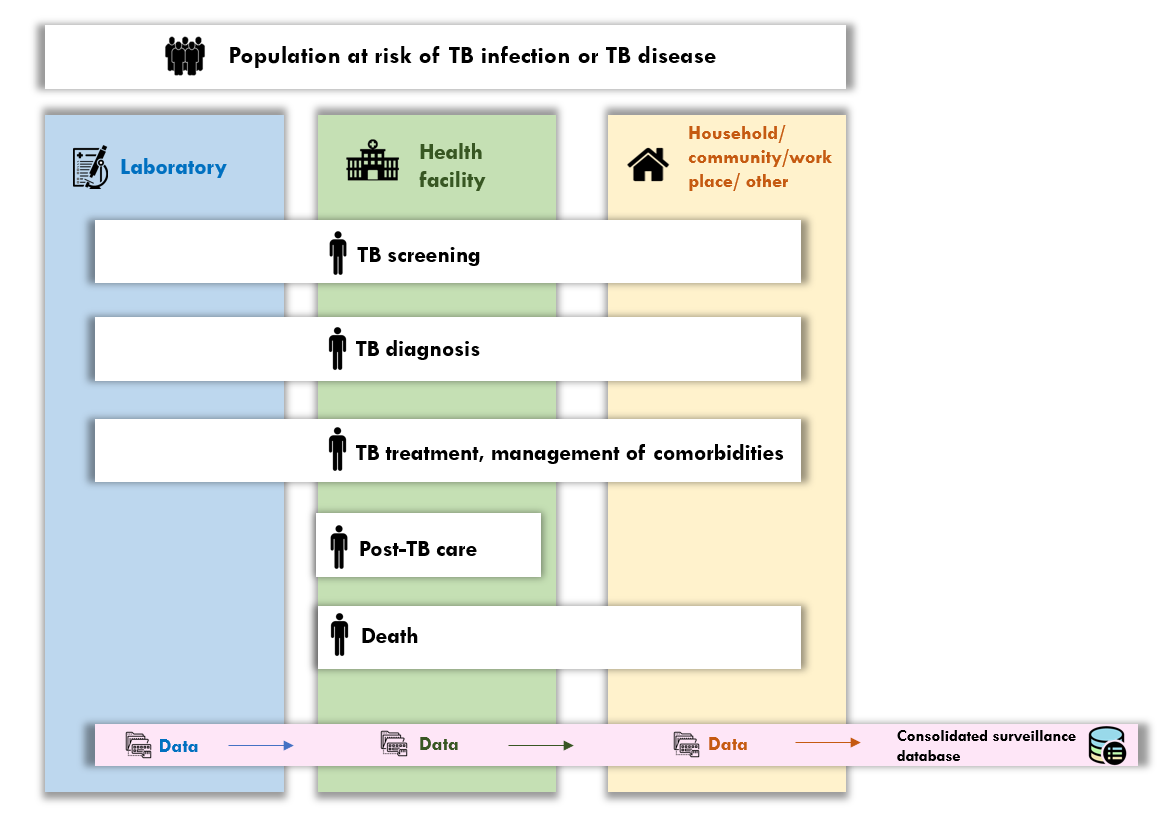
The longer term goal is that all countries are able to rely on a unified case-based digital environment for TB surveillance, along the complete pathway of prevention and care for people at risk of TB infection and TB disease; an illustration is shown in Fig. 3 . This will be supported by WHO standards for metadata, indicators and analytics (via a software-agnostic digital accelerator kit for TB, for countries that would like to develop the environment in the software of their choice), as well as a fully developed environment in DHIS2 (for countries that are looking for an off-the-shelf solution).
The success of case-based digital surveillance for TB in most countries is not just about the availability of technical products (e.g. digital packages, data standards and guidance). Other prerequisites include the necessary infrastructure, a competent core national health information and surveillance team, sufficient staffing and funding, and political commitment to TB data. WHO is currently developing standardized terms of reference for national assessments of readiness to adopt and implement case-based digital TB surveillance, in collaboration with stakeholders including national and local governments, technical agencies, funding agencies and civil society.
Fig. 1 Countries with national, case-based digital surveillance systems for TB, 2020
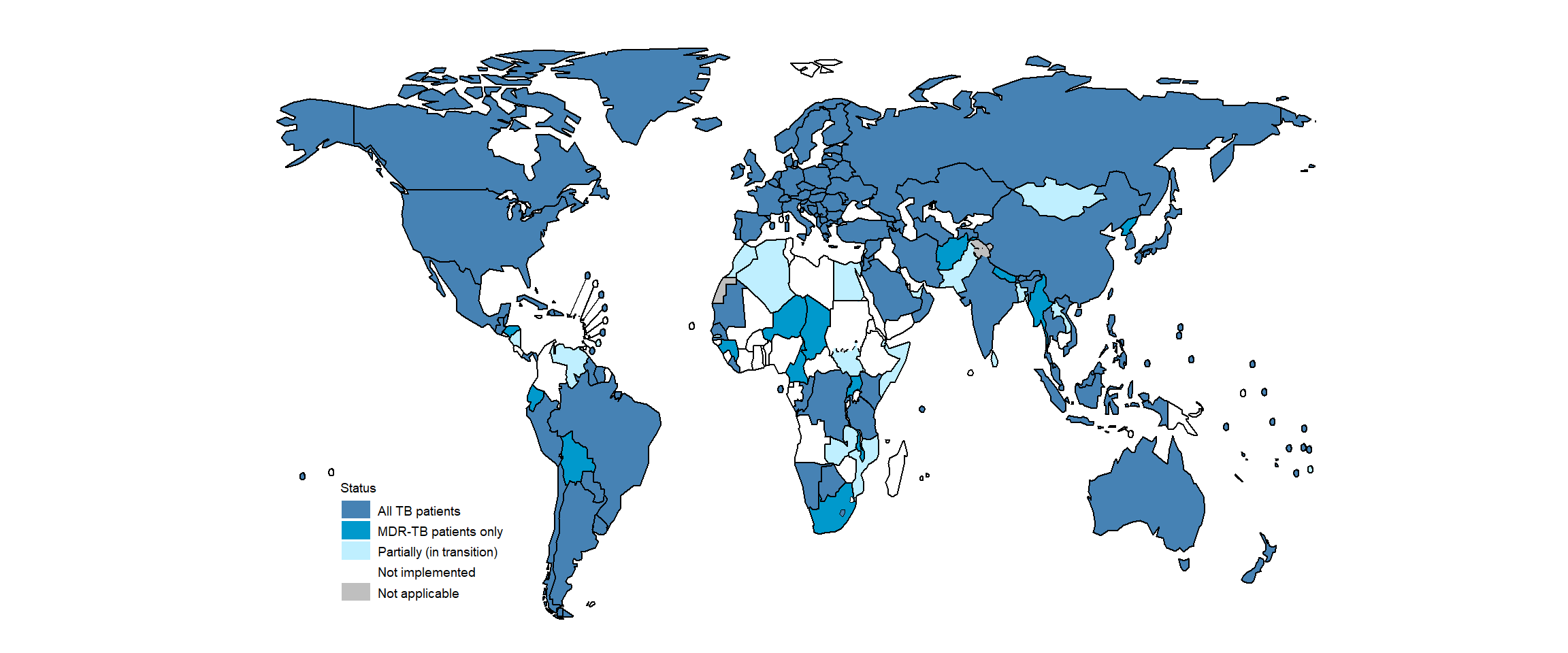
Fig. 2 Global status of implementation and use of the WHO TB DHIS2 packages for health facility and case-based data, 2017–2020

Fig. 3 An illustration of a unified, digital environment for TB surveillance, along the pathway of care
- WHO Tuberculosis Programme: framework for effective TB control. Geneva: World Health Organization; 1994 ( https://apps.who.int/iris/handle/10665/58717 ).
- Revised TB recording and reporting forms and registers - version 2006 (WHO/HTM/TB/2006.373). Geneva: World Health Organization; 2006 ( https://www.who.int/tb/err/rr_final_forms_en.pdf ).
- Definitions and reporting framework for tuberculosis - 2013 revision (updated December 2014 and January 2020). Geneva: World Health Organization; 2013 ( https://apps.who.int/iris/handle/10665/79199 ).
- WHO toolkit for routine health information systems data [website]. Geneva: World Health Organization; 2021. ( https://www.who.int/data/data-collection-tools/health-service-data/toolkit-for-routine-health-information-system-data/modules ).
- Electronic recording and reporting for tuberculosis care and control. Geneva: World Health Organization; 2012 ( https://apps.who.int/iris/handle/10665/44840 ).
- Understanding and using tuberculosis data (WHO/HTM/TB/2014.09). Geneva: World Health Organization Global Task Force on TB Impact Measurement; 2014 ( https://apps.who.int/iris/handle/10665/129942 ).
- Standards and benchmarks for tuberculosis surveillance and vital registration systems: checklist and user guide (WHO/HTM/TB/2014.02). Geneva: World Health Organization; 2014 ( https://apps.who.int/iris/handle/10665/112673 ).
- Metadata package downloads [website]. Geneva: World Health Organization; 2021 ( https://dhis2.org/metadata-package-downloads/ ).
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 01 August 2024
Differential rates of Mycobacterium tuberculosis transmission associate with host–pathogen sympatry
- Matthias I. Gröschel ORCID: orcid.org/0000-0002-2509-3034 1 , 2 , 3 na1 ,
- Francy J. Pérez-Llanos ORCID: orcid.org/0000-0002-9488-0904 4 , 5 , 6 na1 ,
- Roland Diel 7 , 8 ,
- Roger Vargas Jr ORCID: orcid.org/0000-0002-7116-5211 1 ,
- Vincent Escuyer 9 ,
- Kimberlee Musser 9 ,
- Lisa Trieu 10 ,
- Jeanne Sullivan Meissner 10 ,
- Jillian Knorr 10 ,
- Don Klinkenberg ORCID: orcid.org/0000-0002-9449-6873 11 ,
- Peter Kouw 12 ,
- Susanne Homolka ORCID: orcid.org/0000-0003-4972-5638 13 ,
- Wojciech Samek 14 , 15 ,
- Barun Mathema 16 ,
- Dick van Soolingen 11 ,
- Stefan Niemann ORCID: orcid.org/0000-0002-6604-0684 4 , 17 na1 ,
- Shama Desai Ahuja 10 &
- Maha R. Farhat ORCID: orcid.org/0000-0002-3871-5760 1 , 18 na1
Nature Microbiology volume 9 , pages 2113–2127 ( 2024 ) Cite this article
991 Accesses
211 Altmetric
Metrics details
- Bacterial genetics
- Epidemiology
Several human-adapted Mycobacterium tuberculosis complex (Mtbc) lineages exhibit a restricted geographical distribution globally. These lineages are hypothesized to transmit more effectively among sympatric hosts, that is, those that share the same geographical area, though this is yet to be confirmed while controlling for exposure, social networks and disease risk after exposure. Using pathogen genomic and contact tracing data from 2,279 tuberculosis cases linked to 12,749 contacts from three low-incidence cities, we show that geographically restricted Mtbc lineages were less transmissible than lineages that have a widespread global distribution. Allopatric host–pathogen exposure, in which the restricted pathogen and host are from non-overlapping areas, had a 38% decrease in the odds of infection among contacts compared with sympatric exposures. We measure tenfold lower uptake of geographically restricted lineage 6 strains compared with widespread lineage 4 strains in allopatric macrophage infections. We conclude that Mtbc strain–human long-term coexistence has resulted in differential transmissibility of Mtbc lineages and that this differs by human population.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Characterizing tuberculosis transmission dynamics in high-burden urban and rural settings
Assortative social mixing and sex disparities in tuberculosis burden
Thinking clearly about social aspects of infectious disease transmission
Data availability.
The raw sequences were deposited at the European Nucleotide Archive or the Sequence Read Archive at the National Center for Biotechnology Information under BioProject identifiers PRJEB9680 , PRJNA766641 and PRJNA882748 . Accession numbers are listed in Supplementary Table 13 . Source data are provided with this paper.
Code availability
All code used in this study was previously published and is publicly available as cited in Methods . No custom code was developed or used.
Brites, D. & Gagneux, S. Co-evolution of Mycobacterium tuberculosis and Homo sapiens . Immunol. Rev. 264 , 6–24 (2015).
Article CAS PubMed PubMed Central Google Scholar
Stucki, D. et al. Mycobacterium tuberculosis lineage 4 comprises globally distributed and geographically restricted sublineages. Nat. Genet. 48 , 1535–1543 (2016).
Merker, M. et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat. Genet. 47 , 242–249 (2015).
Hirsh, A. E., Tsolaki, A. G., DeRiemer, K., Feldman, M. W. & Small, P. M. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc. Natl Acad. Sci. USA 101 , 4871–4876 (2004).
Demay, C. et al. SITVITWEB—a publicly available international multimarker database for studying Mycobacterium tuberculosis genetic diversity and molecular epidemiology. Infect. Genet. Evol. 12 , 755–766 (2012).
Article CAS PubMed Google Scholar
Wiens, K. E. et al. Global variation in bacterial strains that cause tuberculosis disease: a systematic review and meta-analysis. BMC Med . 16 , 196 (2018).
Article PubMed PubMed Central Google Scholar
Roetzer, A. et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med. 10 , e1001387 (2013).
Holt, K. E. et al. Frequent transmission of the Mycobacterium tuberculosis Beijing lineage and positive selection for the EsxW Beijing variant in Vietnam. Nat. Genet. 50 , 849–856 (2018).
Grosset, J., Rist, S. S. & Meyer, N. Cultural and biochemical characteristics of tubercle bacilli isolated from 230 cases of tuberculosis in Mali. Bull. Int. Union Tuberc. 49 , 177–187 (1974).
Google Scholar
De Jong, B. C. et al. Use of spoligotyping and large sequence polymorphisms to study the population structure of the Mycobacterium tuberculosis complex in a cohort study of consecutive smear-positive tuberculosis cases in the Gambia. J. Clin. Microbiol. 47 , 994–1001 (2009).
Asante-Poku, A. et al. Molecular epidemiology of Mycobacterium africanum in Ghana. BMC Infect. Dis. 16 , 385 (2016).
Chakravarti, A. et al. Indigenous transmission of Mycobacterium africanum in Canada: a case series and cluster analysis. Open Forum Infect. Dis. 6 , ofz088 (2019).
Firdessa, R. et al. Mycobacterial lineages causing pulmonary and extrapulmonary tuberculosis, Ethiopia. Emerg. Infect. Dis. 19 , 460–463 (2013).
Gagneux, S. et al. Variable host–pathogen compatibility in Mycobacterium tuberculosis . Proc. Natl Acad. Sci. USA 103 , 2869–2873 (2006).
Comas, I. et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 45 , 1176–1182 (2013).
Bos, K. I. et al. Pre-Columbian mycobacterial genomes reveal seals as a source of New World human tuberculosis. Nature 514 , 494–497 (2014).
Kay, G. L. et al. Eighteenth-century genomes show that mixed infections were common at time of peak tuberculosis in Europe. Nat. Commun. 6 , 6717 (2015).
Article PubMed Google Scholar
Sabin, S. et al. A seventeenth-century Mycobacterium tuberculosis genome supports a Neolithic emergence of the Mycobacterium tuberculosis complex. Genome Biol. 21 , 201 (2020).
Vågene, Å. J. et al. Geographically dispersed zoonotic tuberculosis in pre-contact South American human populations. Nat. Commun. 13 , 1195 (2022).
Pepperell, C. S. et al. The role of selection in shaping diversity of natural M. tuberculosis populations. PLoS Pathog. 9 , e1003543 (2013).
Reed, M. B. et al. Major Mycobacterium tuberculosis lineages associate with patient country of origin. J. Clin. Microbiol. 47 , 1119–1128 (2009).
Hershberg, R. et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6 , e311 (2008).
Namouchi, A., Didelot, X., Schöck, U., Gicquel, B. & Rocha, E. P. C. After the bottleneck: genome-wide diversification of the Mycobacterium tuberculosis complex by mutation, recombination, and natural selection. Genome Res . 22 , 721–734 (2012).
Nebenzahl-Guimaraes, H., Borgdorff, M. W., Murray, M. B. & van Soolingen, D. A novel approach—the propensity to propagate (PTP) method for controlling for host factors in studying the transmission of Mycobacterium tuberculosis . PLoS ONE 9 , e97816 (2014).
World Health Organization Global Tuberculosis Report 2017 (World Health Organization, 2017).
Freschi, L. et al. Population structure, biogeography and transmissibility of Mycobacterium tuberculosis . Nat. Commun. 12 , 6099 (2021).
Baker, L., Brown, T., Maiden, M. C. & Drobniewski, F. Silent nucleotide polymorphisms and a phylogeny for Mycobacterium tuberculosis . Emerg. Infect. Dis. 10 , 1568–1577 (2004).
Asante-Poku, A. et al. Mycobacterium africanum is associated with patient ethnicity in Ghana. PLoS Negl. Trop. Dis. 9 , e3370 (2015).
Klinkenberg, D., Backer, J. A., Didelot, X., Colijn, C. & Wallinga, J. Simultaneous inference of phylogenetic and transmission trees in infectious disease outbreaks. PLoS Comput. Biol. 13 , e1005495 (2017).
HIV Surveillance Annual Report, 2020 (New York City Department of Health and Mental Hygiene, 2020); https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2020.pdf
Gounder, P. P. et al. Risk for tuberculosis disease among contacts with prior positive tuberculin skin test: a retrospective cohort study, New York City. J. Gen. Intern. Med. 30 , 742–748 (2015).
Getahun, H. et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 46 , 1563–1576 (2015).
Mathema, B. et al. Drivers of tuberculosis transmission. J. Infect. Dis. 216 , S644–S653 (2017).
de Jong, B. C., Antonio, M. & Gagneux, S. Mycobacterium africanum —review of an important cause of human tuberculosis in West Africa. PLoS Negl. Trop. Dis. 4 , e744 (2010).
Coscolla, M. et al. Phylogenomics of Mycobacterium africanum reveals a new lineage and a complex evolutionary history. Microb. Genom. 7 , 000477 (2021).
CAS PubMed PubMed Central Google Scholar
Ruis, C. et al. A lung-specific mutational signature enables inference of viral and bacterial respiratory niche. Microb. Genom. 9 , mgen001018 (2023).
PubMed PubMed Central Google Scholar
Duffy, S. C. et al. Reconsidering Mycobacterium bovis as a proxy for zoonotic tuberculosis: a molecular epidemiological surveillance study. Lancet Microbe 1 , e66–e73 (2020).
Kodaman, N. et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl Acad. Sci. USA 111 , 1455–1460 (2014).
Gygli, S. M. et al. Prisons as ecological drivers of fitness-compensated multidrug-resistant Mycobacterium tuberculosis . Nat. Med. 27 , 1171–1177 (2021).
Shea, J. et al. Comprehensive whole-genome sequencing and reporting of drug resistance profiles on clinical cases of Mycobacterium tuberculosis in New York State. J. Clin. Microbiol. 55 , 1871–1882 (2017).
Diel, R. et al. Accuracy of whole genome sequencing to determine recent tuberculosis transmission: an 11-year population-based study in Hamburg, Germany. Eur. Respir. J . https://doi.org/10.1183/13993003.01154-2019 (2019).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34 , i884–i890 (2018).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://doi.org/10.48550/arXiv.1303.3997 (2013).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9 , e112963 (2014).
Marin, M. et al. Benchmarking the empirical accuracy of short-read sequencing across the M. tuberculosis genome. Bioinformatics 38 , 1781–1787 (2022).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25 , 2078–2079 (2009).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32 , 268–274 (2015).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44 , W242–W245 (2016).
Coll, F. et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat. Commun. 5 , 4812 (2014).
Couvin, D., Reynaud, Y. & Rastogi, N. Two tales: worldwide distribution of Central Asian (CAS) versus ancestral East-African Indian (EAI) lineages of Mycobacterium tuberculosis underlines a remarkable cleavage for phylogeographical, epidemiological and demographical characteristics. PLoS ONE 14 , e0219706 (2019).
Netikul, T., Palittapongarnpim, P., Thawornwattana, Y. & Plitphonganphim, S. Estimation of the global burden of Mycobacterium tuberculosis lineage 1. Infect. Genet. Evol. 91 , 104802 (2021).
O’Neill, M. B. et al. Lineage specific histories of Mycobacterium tuberculosis dispersal in Africa and Eurasia. Mol. Ecol. 28 , 3241–3256 (2019).
Menardo, F. et al. Local adaptation in populations of Mycobacterium tuberculosis endemic to the Indian Ocean Rim. F1000Res 10 , 60 (2021).
Coscolla, M. & Gagneux, S. Consequences of genomic diversity in Mycobacterium tuberculosis . Semin. Immunol. 26 , 431–444 (2014).
Bifani, P. J., Mathema, B., Kurepina, N. E. & Kreiswirth, B. N. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol 10 , 45–52 (2002).
Glynn, J. R., Whiteley, J., Bifani, P. J., Kremer, K. & van Soolingen, D. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis : a systematic review. Emerg. Infect. Dis. 8 , 843–849 (2002).
Cowley, D. et al. Recent and rapid emergence of W-Beijing strains of Mycobacterium tuberculosis in Cape Town, South Africa. Clin. Infect. Dis. 47 , 1252–1259 (2008).
Källenius, G. et al. Evolution and clonal traits of Mycobacterium tuberculosis complex in Guinea-Bissau. J. Clin. Microbiol. 37 , 3872–3878 (1999).
Bouke C. de Jong, Martin Antonio, Timothy Awine, Kunle Ogungbemi, Ype P. de Jong, Sebastien Gagneux, Kathryn DeRiemer, Thierry Zozio, Nalin Rastogi, Martien Borgdorff, Philip C. Hill, and Richard A. AdegbolaUse of spoligotyping and large-sequence polymorphisms to study the population structure of the Mycobacterium tuberculosis complex in a cohort study of consecutive smear positive tuberculosis cases in the Gambia. J Clin Microbiol. 2009 Apr; 47(4): 994–1001
Walker, T. M. et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect. Dis. 13 , 137–146 (2013).
Jombart, T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics 24 , 1403–1405 (2008).
Reback, J. et al. pandas-dev/pandas: Pandas 1.3.5. Zenodo https://doi.org/10.5281/ZENODO.3509134 (2021).
Harris, C. R. et al. Array programming with NumPy. Nature 585 , 357–362 (2020).
R Core Team. R: a language and environment for statistical computing . (R Foundation for Statistical Computing, 2013). URL: https://www.r-project.org/
Wickham, H. et al. Welcome to the Tidyverse. JOSS 4 , 1686 (2019).
Article Google Scholar
Finch, W. H., Finch, M. E. H. & Singh, M. Data imputation algorithms for mixed variable types in large scale educational assessment: a comparison of random forest, multivariate imputation using chained equations, and MICE with recursive partitioning. Int. J. Quant. Res. Educ. 3 , 129 (2016).
Download references
Acknowledgements
We thank P. Lapierre from the Wadsworth Center, New York State Department of Health, Albany, New York, and the Wadsworth Center Applied Genomics Technology Cluster for whole-genome sequencing and data transfer. We acknowledge H. de Neeling, H. Schimmel and E. Slump from the National Institute for Public Health and the Environment, Bilthoven, the Netherlands. We thank V. Dreyer and T. Kohl for transferring sequence data, M. Hein and T. Scholzen from the Flow Cytometry Core, F. Daduna for participant recruitment and D. Beyer and S. Maaß for technical assistance, all at the Research Center Borstel. This work was funded by National Institutes of Health/National Institute of Allergy and Infectious Diseases R21 AI154089 to M.R.F.; the German Research Foundation (GR5643/1-1) to M.I.G.; the BIH Charité Junior Digital Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin; the Berlin Institute of Health at Charité (BIH) to M.I.G.; the Leibniz Science Campus EvoLUNG (Evolutionary Medicine of the Lung; https://evolung.fz-borstel.de/ ) grant number W47/2019 to F.J.P.-L., S.N. and S.H.; the German Research Foundation under Germany’s Excellence Strategy–EXC 2167 Precision Medicine in Inflammation; and the German Ministry of Education and Research (BMBF) for the German Center of Infection Research (DZIF) to S.N. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
These authors contributed equally: Matthias I. Gröschel, Francy J. Pérez-Llanos, Stefan Niemann, Maha R. Farhat.
Authors and Affiliations
Department of Biomedical Informatics, Harvard Medical School, Boston, MA, USA
Matthias I. Gröschel, Roger Vargas Jr & Maha R. Farhat
Department of Infectious Diseases and Respiratory Medicine, Charité—Universitätsmedizin Berlin, Berlin, Germany
Matthias I. Gröschel
Berlin Institute of Health at Charité—Universitätsmedizin Berlin, Berlin, Germany
Molecular and Experimental Mycobacteriology, Research Center Borstel, Borstel, Germany
Francy J. Pérez-Llanos & Stefan Niemann
West German Genome Center, Heinrich Heine University Düsseldorf, Düsseldorf, Germany
Francy J. Pérez-Llanos
Institute of Human Genetics, The University Hospital of Düsseldorf, Düsseldorf, Germany
Institute for Epidemiology, University Medical Hospital Schleswig-Holstein, Kiel, Germany
Roland Diel
Lungenclinic Grosshansdorf, Airway Research Center North (ARCN), Member of the German Center for Lung Research (DZL), Grosshansdorf, Germany
Wadsworth Center, New York State Department of Health, Albany, NY, USA
Vincent Escuyer & Kimberlee Musser
New York City Department of Health and Mental Hygiene, New York, NY, USA
Lisa Trieu, Jeanne Sullivan Meissner, Jillian Knorr & Shama Desai Ahuja
Center for Infectious Disease Control, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands
Don Klinkenberg & Dick van Soolingen
Department of Tuberculosis, Public Health Service, Amsterdam, The Netherlands
Diagnostic Mycobacteriology, National and Supranational Reference Center for Mycobacteria, Research Center Borstel, Borstel, Germany
Susanne Homolka
Department of Electrical Engineering and Computer Science, Technical University Berlin, Berlin, Germany
Wojciech Samek
Department of Artificial Intelligence, Fraunhofer Heinrich Hertz Institute, Berlin, Germany
Mailman School of Public Health, Columbia University, New York City, NY, USA
Barun Mathema
German Center for Infection Research, Partner Site Hamburg–Lübeck–Borstel–Riems, Borstel, Germany
Stefan Niemann
Division of Pulmonary and Critical Care Medicine, Massachusetts General Hospital, Boston, MA, USA
Maha R. Farhat
You can also search for this author in PubMed Google Scholar
Contributions
M.R.F. and M.I.G. conceived the idea for the epidemiological analysis. S.N., S.H. and F.J.P.-L. conceived the idea for the in vitro experiments. M.R.F. supervised the project. M.I.G. performed data curation and data analysis. M.I.G. and M.R.F. wrote the first draft. F.J.P.-L. performed data curation and data analysis. R.V.Jr. and D.K. analysed the data. L.T., P.K. and R.D. carried out data acquisition. V.E., K.M., J.S.M., S.H., D.v.S., S.D.A. and S.N. supervised data acquisition and curation. W.S. and B.M. critically reviewed the drafts. All authors reviewed the draft and assisted in the preparation of the paper.
Corresponding authors
Correspondence to Matthias I. Gröschel or Maha R. Farhat .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Microbiology thanks Sebastien Gagneux, Stephen Gordon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1 genetic characteristics of m. tuberculosis complex strain..
a) Violin plot of the terminal branch lengths of the included Mtbc genetic lineages. The overlayed box plots display the median, the first and third quartile, and the horizontal lines represent the upper and lower values of the data. L1 = 523, L2 widespread = 707, L2 restricted = 75, L3 = 681, L4 widespread = 2,494, L4 restricted = 220, L5 = 17, L6 = 27 strains, respectively. b) Proportions of strains in clusters based on several different Single Nucleotide Substitution (SNS) thresholds by genetic lineage and site. L = Lineage, SNP = Single Nucleotide Polymorphism, NYC = New York City, NL = The Netherlands, HH = Hamburg, L2 restricted includes sub-lineages 2.1., 2.2.2., and 2.2.1.1.2, L4 restricted sub-lineages include 4.11, 4.2.1.1, 4.3.i2, 4.5, and 4.6.2.2. L2 widespread refers to sub-lineages 2.2.1, 2.2.1.1, 2.2.1.1.1, 2.2.1.1.1i1, 2.2.1.1.1.i2, 2.2.1.1.1.i3, 2.2.1.2, L4 to all other L4 sub-lineages (see Methods ).
Source data
Extended data fig. 2 relationship of index case self-reported ancestry and human-adapted m. tuberculosis complex lineage..
a) Bar plot detailing the proportions of isolation country and Mtbc lineage in a global sample of 25,243 strains. b) Adjusted odds ratios estimated for the variable contact allopatry using different co-localization or sympatry assumptions from multivariate Generalized Estimation Equation (GEE) models (see Fig. 3f in main text). No effect for L4 widespread is shown. L2 restricted includes sub-lineages 2.1., 2.2.2 and 2.2.1.1.2, L4 restricted sub-lineages include 4.11, 4.2.1.1, 4.3.i2, 4.5, and 4.6.2.2. L2 widespread refers to sub-lineages 2.2.1, 2.2.1.1, 2.2.1.1.1, 2.2.1.1.1i1, 2.2.1.1.1.i2, 2.2.1.1.1.i3, 2.2.1.2, L4 to all other L4 sub-lineages (see Methods ). The bars represent the effect estimates from the GEE models with 95% confidence intervals. N = 2,556 contacts.
Extended Data Fig. 3 Comparison of the inflammatory response induced by M. tuberculosis complex L6 (a-b) and L4 (c-d) strains in human monocyte derived macrophages (MDMs) based on their self reported ancestry colocalizing with L6 at 24 (a-c) and 96 (b-d) hours post-infection.
Six human inflammatory cytokines-chemokines were screened using LEGENDplex. The stacked bars represent the mean production of IL-1ß, TNF-α, MCP-1, IL-6, IL-8, and IL-18. Each bar represents three donors colocalizing with strains of Lineage 6 (Yes [Nigerian, Cameroonian, Ghanaian]) and no colocalizing with strains of Lineage 6 (No [German donors]). PBS, Macrophage Infection Media (MIM), and supernatants from not infected MDMs were used as controls. The MIM values were subtracted from the not infected and infected MDMs. Mean, standard error of the mean, and significant statistical results (* P < 0.05; ** P < 0.01; *** P < 0.001 and **** P < 0.0001) are shown. Statistical results were calculated based on Two-way ANOVA multiple comparison with Bonferroni correction. Data were obtained from six independent infection experiments (three for each donor group). L6, Lineage 6; L4, Lineage 4; hpi, hours post-infection.
Extended Data Fig. 4 Cytokine response of human macrophages of donors with self-reported ancestry to Europe to distinct Mycobacterium tuberculosis complex strains.
This assay was conducted on cell culture supernatants collected from MDMs that were infected with 3 representative strains of L4 and L6, and also no infected MDMs. The infection was carried out with an MOI ~ 1:1, and the supernatants were collected at 24 and 96 hours post-infection (hpi). Three infection macrophage wells were tested per strain, time point, and donor. 13 human inflammatory cytokines-chemokines were screened using LEGENDplex. The production of six detected cytokines-chemokines is depicted in the figure, namely IL-1ß (a), TNF-α (b), MCP-1 (c), IL-6 (d), IL-8 (e) and IL-18 (f). The protein concentration in pg/mL was plotted on the y-axis, while the x-axis represented the controls (PBS and no-infected [NI] at 24 hpi and 96 hpi) and experimental conditions (infected with L4, and L6 at 24 hpi and 96 hpi). The stacked bars compared the mean production of these cytokines-chemokines at 24 hpi and 96 hpi within a lineage and across lineages. Each lineage is represented by three strains (three dots), and each strain comprises the averaged values of three donors. PBS, Macrophage Infection Media (MIM), and non-infected cells were used as controls. The MIM values were subtracted from no-infected and infected wells. The mean, standard error of the mean, and statistical results (ns >0.05; * P < 0.05; ** P < 0.01; *** < 0.001 and **** P < 0.0001) are depicted in the figures. Data were obtained from three independent infection experiments. The statistical results shown in the figures are two-sided p-values based on an unpaired t -test among both time points within the same lineage strain and on one-way ANOVA with Bonferroni post hoc test correction-multiple comparisons across distinct lineages at 24 hpi and 96 hpi. MDMs, Monocyte Blood Derived Macrophages; ns, not significant; NI, not-infected; L4, Lineage 4; L6, Lineage 6; CFU, Colony Forming Unit; MOI, Multiplicity of Infection; h, hours.
Extended Data Fig. 5 Cytokine response of human macrophages of donors with self-reported ancestry to Ghana, Cameroon, and Nigeria to distinct Mycobacterium tuberculosis complex strains.
This assay was conducted on cell culture supernatants collected from MDMs that were infected with 3 representative strains of L4 and L6, and also no infected MDMs. The infection was carried out with an MOI ~ 1:1, and the supernatants were collected at 24 and 96 hours post-infection (hpi). Three infection macrophage wells were tested per strain, time point, and donor. 13 human inflammatory cytokines-chemokines were screened using LEGENDplex. The production of six detected cytokines-chemokines is depicted in the figure, namely IL-1ß (a), TNF-α (b), MCP-1 (c), IL-6 (d), IL-8 (e) and IL-18 (f). The protein concentration in pg/mL was plotted on the y-axis, while the x-axis represented the controls (PBS and no-infected [NI] at 24 hpi and 96 hpi) and experimental conditions (infected with L4, and L6 at 24 hpi and 96 hpi). The stacked bars compared the mean production of these cytokines-chemokines at 24 hpi and 96 hpi within a lineage and across lineages. Each lineage is represented by three strains (three dots), and each strain comprises the averaged values of three donors. PBS, Macrophage Infection Media (MIM), and no-infected cells were used as controls. The MIM values were previously subtracted from no-infected and infected wells. The mean, standard error of the mean, and statistical results (ns, P >0.05; * P < 0.05; ** P < 0.01; *** P < 0.001 and **** P < 0.0001) are depicted in the figures. Data were obtained from three independent infection experiments. The statistical results shown in the figures are two-sided p-values based on an unpaired t -test among both time points within the same lineage strain and on one-way ANOVA with Bonferroni post hoc test correction-multiple comparisons across distinct lineages at 24 hpi and 96 hpi. MDMs, Monocyte Blood Derived Macrophages; ns, not significant; NI, not-infected; L4, Lineage 4; L6, Lineage 6; CFU, Colony Forming Unit; MOI, Multiplicity of Infection; h, hours.
Extended Data Fig. 6 Comparison of tuberculosis index case and social contact group characteristics.
a) Dot plot of the index case age (x-axis) versus mean contact group age (y-axis) for each of the included cities; b) Dot plot of the No. of M. tuberculosis infections per contact group (x-axis) and the size of the contact group (y-axis). A linear regression line is overlayed with 95% confidence intervals.
Supplementary information
Supplementary information.
Legends for Extended Data Figs. 1–6, Supplementary Figs. 1–7 and Supplementary Tables 1–12.
Reporting Summary
Supplementary table 13.
Accession codes for sequence data used in this study.
Source Data Fig. 1
Source data fig. 2.
Statistical source data.
Source Data Fig. 3
Source data fig. 4 and source data extended data fig. 3–5, source data extended data fig. 1, source data extended data fig. 2, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Gröschel, M.I., Pérez-Llanos, F.J., Diel, R. et al. Differential rates of Mycobacterium tuberculosis transmission associate with host–pathogen sympatry. Nat Microbiol 9 , 2113–2127 (2024). https://doi.org/10.1038/s41564-024-01758-y
Download citation
Received : 31 October 2023
Accepted : 12 June 2024
Published : 01 August 2024
Issue Date : August 2024
DOI : https://doi.org/10.1038/s41564-024-01758-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

- Entertainment
- Life & Style

To enjoy additional benefits
CONNECT WITH US

39% TB cases found during Tamil Nadu TB survey had no symptoms Premium
The t.n. tb programme should prioritise the use of chest x-rays for earlier detection of cases and should upscale molecular tests, the study authors recommend.
Updated - August 05, 2024 11:19 am IST
Published - August 03, 2024 09:00 pm IST

The first-of-its-kind TB prevalence cross-sectional survey was carried out among individuals aged older than 15 years across Tamil Nadu from February 2021 to July 2022. Based on the survey findings, the authors of the study from the National Institute for Research in Tuberculosis (ICMR-NIRT), Chennai have recommended that the State TB programme should prioritise the use of chest X-rays for earlier detection of cases and to cut the transmission chain, and should upscale molecular tests to increase the yield. The results were published recently in The International Journal of Tuberculosis and Lung Disease .
In a cross-sectional survey, 130,932 participants consented to participate. Among them, 130,914 underwent symptom screening and 125,870 underwent both symptom screening and chest X-ray examination. Those with symptoms of TB and/or abnormal chest X-rays were tested for TB using Xpert, smear microscopy, and liquid culture. The survey identified 244 microbiologically confirmed TB cases. Among the 244 patients with TB, only 54.5% (133 people) reported having symptoms of TB, while 92.6% (224 people) had chest X-ray abnormalities.
Among the 244 TB cases detected, molecular test (CBNAAT) correctly detected 224 (91.8%) cases, while smear microscopy was able to detect only 123 (50.40%) cases. Even among the 115 symptomatic TB cases, 89% (102) were diagnosed by CBNAAT compared with 58 (50%) who were diagnosed based on smear microscopy. The Tamil Nadu TB programme, which over-relies on smear microscopy, must scale up molecular tests, the authors write.
The most important finding from the survey is that 39% (94 people) of the TB cases detected had no TB symptoms, otherwise called subclinical TB, which would have been missed if a chest X-ray had not been used. All 94 people with TB, who were initially picked up based on chest X-ray abnormalities and subjected to sputum examination, were found to be bacteriologically positive, thus confirming TB disease. This highlights the importance of using chest X-ray for screening even in people who do not exhibit any symptoms. Currently, the TB programme in Tamil Nadu offers chest X-rays only for those with symptoms. Based on the survey findings, the State should prioritise the use of chest X-rays for earlier case detection and increase the yield.
Even active case finding of high-risk/vulnerable groups across India screens people for presumptive TB symptoms/signs, leaving out a huge chunk of people with subclinical TB. As per the India TB Report 2022, 22.1 crore individuals in India were screened for presumptive TB as part of active case-finding. Of these, only 48,329 (2.5%) were diagnosed, resulting in a low yield of just 22 per 100,000 population. Considering that TB incidence in 2022 was 199 per 100,000, active case finding, which is a targeted approach to find more cases in vulnerable populations, should have produced a higher yield. The use of chest X-ray as a preliminary screening tool along with molecular diagnostics will surely help in better detection of cases.
That 39% of TB cases detected had no symptoms is not surprising. As per the National TB Prevalence Survey report (2019-2021), 42.6 % of the TB cases, which were bacteriologically positive but did not exhibit any TB symptoms, would have been missed if chest X-ray was not included as an additional screening tool.
Besides, picking up more people with TB when chest X-ray is used, early detection of subclinical TB cases will greatly help in cutting the transmission chain. According to a March 2024 paper in the journal Lancet Infectious Diseases , a meta-analysis of surveys from a few high TB-burden countries found 27.7% of people with TB had no TB symptoms. The paper says that the majority of people with pulmonary TB in the community do not cough. “A quarter of those not reporting any cough have positive sputum smears, suggesting infectiousness. In high-incidence settings, subclinical tuberculosis could contribute considerably to the tuberculosis burden and transmission,” the paper says. In India and other high-burden countries, subclinical TB may be hiding a higher prevalence of the disease.
Even when people do not exhibit symptoms, they can still have the high bacillary loads typically associated with transmission, according to a 2021 paper in the American Journal of Respiratory and Critical Care Medicine . It also says that though cough is associated with higher infectiousness, cough is not necessary for transmission. “Subclinical TB can potentially drive a substantial fraction of transmission on a population level because of its high prevalence and long duration,” it says. Although cough expels large quantities of droplets leading to increased transmission risk, respiratory droplets can also be expelled without cough such as during singing, talking, and tidal breathing, the paper says.
Across the globe, TB incidence has been dropping more slowly compared with TB deaths suggesting that all TB measures have been less effective in stopping transmission. According to the 2021 paper, one explanation for the slower reduction of TB incidence might be that people with “subclinical TB may be the source of a large fraction of ongoing TB transmission”.
Related Topics
tuberculosis / health / communicable diseases / disease prevention / medical research
Top News Today
- Access 10 free stories every month
- Save stories to read later
- Access to comment on every story
- Sign-up/manage your newsletter subscriptions with a single click
- Get notified by email for early access to discounts & offers on our products
Terms & conditions | Institutional Subscriber
Comments have to be in English, and in full sentences. They cannot be abusive or personal. Please abide by our community guidelines for posting your comments.
We have migrated to a new commenting platform. If you are already a registered user of The Hindu and logged in, you may continue to engage with our articles. If you do not have an account please register and login to post comments. Users can access their older comments by logging into their accounts on Vuukle.
- DOI: 10.5195/ijms.2024.2147
- Corpus ID: 271633578
Disseminated Tuberculosis with Testes Involvement: An Intriguing Case Report
- Arnab Kundu , Ramanuj Mukherjee , +1 author Gouri Mukhopadhyay
- Published in International Journal of… 9 July 2024
Figures and Tables from this paper

One Citation
Transforming toxic research cultures: protecting the future of medical students and early career researchers – part i, 6 references, testicular cancer presenting as disseminated tuberculosis: a case report, review of literature on disseminated tuberculosis with emphasis on the focused diagnostic workup, testicular tuberculosis: two rare case report, case 91: tuberculous epididymo-orchitis., tb or not tb: a case of isolated testicular tb with scrotal involvement, testicular tuberculosis, related papers.
Showing 1 through 3 of 0 Related Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Emerg Infect Dis
- v.8(11); 2002 Nov
Two Cases of Pulmonary Tuberculosis Caused by Mycobacterium tuberculosis subsp. canetti
Jean miltgen.
* Hôpital d’instruction des armées Laveran, Marseille, France
Marc Morillon
Jean-louis koeck.
† Hôpital d’instruction des armées Val de Grâce, Paris, France
Anne Varnerot
‡ Institut Pasteur, Paris, France
Jean-François Briant
Gilbert nguyen, denis verrot, daniel bonnet, véronique vincent.
We identified an unusual strain of mycobacteria from two patients with pulmonary tuberculosis by its smooth, glossy morphotype and, primarily, its genotypic characteristics. Spoligotyping and restriction fragment length polymorphism typing were carried out with the insertion sequence IS6110 patterns. All known cases of tuberculosis caused by Mycobacterium canetti have been contracted in the Horn of Africa.
The Mycobacterium tuberculosis complex includes the following mycobacteria, which are characterized by a slow growing rate: M. tuberculosis, M. africanum, M. bovis, and M. microti ( 1 ). In recently published reports of two cases of lymphatic node tuberculosis (TB), the strains were recognized as belonging to a new taxon of M. tuberculosis ( 2 , 3 ). These isolates were characterized by a highly particular growing pattern, and the colonies appeared smooth and glossy. A complete genetic study of these strains led to their integration into the M. tuberculosis complex. This strain, identified as M. tuberculosis subsp. canetti or, more simply, M. canetti, was first isolated in 1969 by Georges Canetti from a French farmer. The strain was preserved at the Pasteur Institute where its antigenic pattern was studied extensively. We report two cases of pulmonary TB caused by this strain. The two patients had also lived in East Africa.
In September 1998, a 36-year-old male soldier in the French Foreign Legion with hemoptysis was sent back to France from Djibouti. He expectorated bloody sputum after running and on a few other occasions. His medical history was not unusual. When the patient was hospitalized, 2 weeks after the initial symptoms, he began to experience progressive fatigue. He did not experience fever, weight loss, night sweats, anorexia, cough, dyspnea, or chest pain, and did not produce sputum.
Results of the clinical examination were normal. The Mantoux test, performed with 10 IU of purified tuberculin (Aventis-Pasteur-MSD, Lyon, France), yielded a maximum transverse diameter of induration of 15 mm. Laboratory values were normal ( Table ). The chest X-ray showed a triangular consolidation of the left upper lobe with blurred limits and small cavitary lesions. No other contiguous mediastinohilar anomalies were visible. A computed tomographic scan confirmed the cavitary syndrome: three excavated nodular images showed radiating spicules within a micronodular infiltrate. Bronchoscopy showed a moderate inflammation of airway mucosa, especially in the left upper lobe. Biopsy specimens exhibited nonspecific inflammation.
| Laboratory test | Patient 1 | Patient 2 |
|---|---|---|
| Sedimentation rate (mm) | 3 | 3 |
| C-reactive protein (mg/L) | 7.3 | 4.28 |
| Fibrinogen (g/L) | 3.64 | 6.3 |
| Blood count | ||
| Hemoglobin (g/dL) | 16.8 | 14.1 |
| Platelets (x109/L) | 194 | 274 |
| White cells (x109/L) | 10.10 | 9.54 |
| Neutrophils (%) | 66.2 | 69.4 |
| Eosinophils (%) | 2 | 8.6 |
| Lymphocytes (%) | 21.3 | 17.2 |
| Basophils (%) | 0.6 | 0.9 |
| Monocytes (%) | 9.9 | 3.9 |
| Aspartate aminotransferase (U/L) | 21 | 16 |
| Alanine aminotransferase (U/L) | 19 | 23 |
| Creatinine (µmol/L) | 89 | 97 |
| Glucose (mmol/L) | 4.7 | 4.4 |
A bronchial washing smear from the left upper lobe was positive for acid-fast bacilli. Serologic tests for HIV-1 and HIV-2 were negative. No evidence of disease was found elsewhere; the patient did not experience bone pain. Results of neurologic and ophthalmologic examinations were normal; no lymphadenopathy or hepatosplenomegaly were found and the genitalia were normal. Auscultation revealed no pericardial fremitus; no ascitic fluid was detected. The urinary sediment contained <1,000 red blood cells/L and <5,000 leukocytes/L. Antituberculosis chemotherapy was begun with four drugs: rifampicin, isoniazid, ethambutol, and pyrazinamide. Cultures revealed a strain identified as M. tuberculosis subsp. canetti that was susceptible to all primary antituberculous drugs. Therefore, rifampicin and isoniazid were continued for 3 more months for a total treatment period of 6 months. The patient’s response to treatment was favorable, and he remained asymptomatic.
A 55-year-old male soldier in the French Foreign Legion, who returned from Djibouti, was hospitalized in September 1999 after his chest x-ray showed abnormal findings. He was a nurse and had been occasionally in charge at the Djibouti Hospital for 2 years. His medical history was unremarkable. Eight months before he returned to France, he experienced asthenia, anorexia, and a weight loss of 3 kg. The symptoms resolved spontaneously after 2 months, and he had been asymptomatic since then. He had no history of cough, sputum production, hemoptysis, dyspnea, fever, or night sweats.
Results of a clinical examination and of laboratory studies were normal ( Table ), except for hypereosinophilia. Serologic tests for schistosomiasis, hydatidosis, distomiasis, amebiasis, toxocariasis, and trichinosis were negative, and parasites were not found in stool samples. Thoracic radiographs performed when he came back from Djibouti showed parenchymal consolidation of the right upper lobe with small cavities. Sputum was not produced. A gastric aspirate smear was negative for acid-fast bacilli, and a bronchial aspiration smear was positive for acid-fast bacilli. HIV serology was negative, and no other site of the infection was found. Drug therapy was initiated with rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months. Cultures of bronchial aspirates were positive within 14 days; later, cultures of two gastric aspirates were positive for acid-fast bacilli. An M. tuberculosis subsp. canetti isolate was identified, which was susceptible to all primary antituberculous drugs. The treatment was then extended for 4 months with rifampicin and isoniazid. The patient's response to treatment was favorable.
The following methods were used to identify the etiologic agent. First, the samples were decontaminated with N-acetyl-L-cysteine/NaOH. Acid-fast bacilli were detected by auramine staining, the positive smears also were stained with Ziehl-Nielsen stain. The samples were then seeded onto Löwenstein-Jensen and Coletsos slants and also into a liquid system, the BBL Mycobacterial Growth Indicator Tube (MGIT, BD Diagnostic Systems, Sparks, MD).
The mycobacteria were identified by using a specific DNA probe (Gen-Probe, Gen-Probe Incorporated, San Diego, CA) and by performing the usual biochemical tests (nitrate reduction, 68°C catalase resistance, niacin production).
The Pasteur Institute of Paris used two methods for typing: restriction fragment length polymorphism (RFLP) analysis and spoligotyping. In RFLP analysis, after digestion of the M. tuberculosis strain's genomic DNA with PvuII restriction enzyme and agarose gel migration, the DNA was transferred on a membrane, according to the Southern method, and then hybridized with an insertion sequence IS6110 probe ( 4 ). In the spoligotyping method, after DNA direct repeat amplification, the labeled polymerase chain reaction product was used as a probe to hybridize with 43 synthetic spacer oligonucleotides (DNA sequences derived from the direct repeat [DR] region of M. tuberculosis, H37Rv and M. bovis BCG P3), which were attached to a carrier membrane ( 5 ). The sensitivity to antituberculous drugs was determined by the indirect proportion method.
MGIT results were positive for the two cultures in 9 and 12 days, respectively. On Löwenstein-Jensen slants, the cultures were positive in 12 and 14 days, respectively. The white, smooth, and glossy colonies were characteristic of M. tuberculosis subsp. canetti ( Figure 1 ). The two strains had the same phenotypic and genotypic pattern; 68°C catalase was negative, and they reduced nitrate, as do other M. tuberculosis species, but they did not produce niacin. The DNA probe, Gen-Probe, confirmed that these strains belonged to the M. tuberculosis complex.

Colony morphology on Löwenstein-Jensen slants, showing M. canetti and M. tuberculosis strains. (A) Colonies of M. tuberculosis are rough, thick, wrinkled, have an irregular margin, and are faintly buff-colored. (B) M. canetti exhibits smooth, white and glossy colonies.
These strains contained two copies of IS6110. Spoligotyping showed that they shared only 2 of the 43 oligonucleotides reproducing the spacer DNA sequences of M. tuberculosis, H37Rv and M. bovis BCG P3. This profile is characteristic of M. tuberculosis subsp. canetti ( Figure 2 ).

(A) IS6110 hybridization patterns of PvuII-digested genomic DNA. Lane 1, Mycobacterium tuberculosis Mt 14323 (reference strain). Lane 2, M. canetti strain NZM 217/94. Lanes 3 and 4, the strains isolated from French legionnaires with pulmonary tuberculosis (TB). (B) Spoligotyping patterns. Lane 1, M. tuberculosis H37Rv (reference strain). Lane 2, M. canetti strain NZM 217/94. Lanes 3 and 4, the strains isolated from French legionnaires with pulmonary TB.
In 1997, van Soolingen reported a case of lymph node TB in a 2-year-old Somali child on the child’s arrival in the Netherlands in 1993 ( 2 ). In 1998, Pfyffer described abdominal lymphatic TB in a 56-year-old Swiss man (who lived in Kenya) with stage C2 HIV infection ( 3 ). These strains of M. canetti (So93 from the Somali child and NZM 217/94 from the Swiss man) have been studied extensively. In culture they grow faster than other strains in the M. tuberculosis complex. The So93 strain expands by one rough colony for every 500 smooth colonies. They appear smooth, white, and glossy because of the high amount of lipooligosaccharides in the membrane ( 6 ); the So93 rough colonies lack this amount ( 2 ).
Two copies of the IS6110 insertion sequence were found in the NZM 217/94 and So93 genome. This fingerprint matched none of the 5,000 other strains preserved in the laboratory of van Soolingen (Bilthoven, the Netherlands) ( 2 ). The strains we observed also showed two copies of IS6110.
So93, NZM 217/94, and our two strains share only 2 of 43 identical repeated sequences that have been observed by spoligotyping. Study of the IS6110 RFLP patterns and of the spacer DNA sequences of the DR locus confirmed that M. tuberculosis, M. bovis, M. africanum, M. microti, and M. canetti represent a closely related group of mycobacteria that are clearly distinct from other mycobacterial species. In the M. tuberculosis complex, M. canetti appears to be the most divergent strain ( 2 ).
We believe that this is the first published report of pulmonary disease caused by M. canetti. Our two cases confirm that M. canetti is able to involve lungs, like any other other member of the M. tuberculosis complex and is able to affect immunocompetent subjects. The clinical features of these two pulmonary cases of TB caused by M. canetti are not specific.
TB caused by M. canetti appears to be an emerging disease in the Horn of Africa. A history of a visit to the region should cause this strain to be considered promptly. As travel to this area becomes more frequent, and mycobacterial identification techniques improve, the number of diagnosed cases will likely increase.
Acknowledgments
We thank Michel Fabre for the photographs and Jan Eskandari for his translation of this article.
Dr. Miltgen is assistant head of the Pneumology Department at the Hôpital d’Instruction des Armées of Marseilles, where he specializes in tropical diseases.
Suggested citation for this article: Miltgen J, Morrillon M, Koeck J-L, Varnerot A, Briant J-F, Nguyen G. Two cases of pulmonary tuberculosis caused by Mycobacterium tuberculosis subsp. canetti. Emerg Infect Dis [serial online] 2002 Nov [date cited]. Available from http://www.cdc.gov/ncidod/EID/vol8no11/02-0017.htm
Feasibility of eliminating tuberculosis by shortening the diagnostic delay: A retrospective analysis and modelling study in China during the pre-COVID-19 era
- Chen, Qiuping
- Guo, Yichao
- Yu, Shanshan
- Li, Kangguo
- Gavotte, Laurent
- Frutos, Roger
- Chen, Tianmu
Delays in the diagnosis and treatment of pulmonary tuberculosis (PTB) can increase the risk of transmission, thereby posing a significant risk to public health. Early diagnosis is considered to play a crucial role in eliminating TB. Rapid testing, active case finding, and health education are effective strategies for reducing tuberculosis diagnosis delays (TDDs). This study aimed to quantitatively compare the impact of reducing the TDD on incidence rates among student and non-student groups, thus exploring the efficacy of shortening the TDD for ending the TB epidemic and providing a reference for achieving the target incidence rate for ending TB. We used unsupervised hierarchical clustering analysis and non-parametric tests to characterize the epidemiological characteristics of TDD. Additionally, a dynamic transmission model was used to quantify the impact of shortening the TDD on the incidence rates of TB among the two groups. There was an initial increase in the TDD, followed by a decrease. Longer TDDs were observed in the northeastern region of China. Farmers, middle and high school students, middle-aged, elderly individuals and males exhibited relatively longer TDDs. A significant reduction in the incidence rate of PTB was observed when the TDD was decreased by 50 %. However, only reducing the TDD among non-students could achieve the goal of ending TB (i.e., achieving a minimum reduction of 63.00 %). TDD remains a serious risk to public health, and non-students were shown to experience longer TDD. Shortening the TDD is crucial for reducing the incidence rates of TB, especially among non-students. It is essential to develop a highly sensitive and effective system for eliminating TB among non-students.
- Diagnostic delays;
- Pulmonary tuberculosis;
- Dynamic model;
- End tuberculosis;
- Active case finding;
- Systematic Review
- Open access
- Published: 07 August 2024
Prevalence, incidence, and case fatality of tuberculous meningitis in adults living with HIV: a systematic review and meta-analysis
- Xue Chen 1 , 2 na1 ,
- Jiaqi Wei 1 na1 ,
- Mei Zhang 1 na1 ,
- Meixin Ren 1 ,
- Miaotian Cai 3 ,
- Yulin Zhang 3 , 4 &
- Tong Zhang 1
BMC Public Health volume 24 , Article number: 2145 ( 2024 ) Cite this article
181 Accesses
Metrics details
Tuberculous meningitis (TBM) emerges as a grave complication of tuberculosis in people living with HIV (PLWH). The diagnosis and treatment of TBM pose significant challenges, leading to elevated mortality rates. To comprehensively grasp the epidemiological landscape of TBM in PLWH, a systematic review and meta-analysis were meticulously undertaken.
We performed a comprehensive search in PubMed, Embase, and Web of Science from database inception to September 19th, 2023, with no limitations on the publication type. The search terms were HIV/AIDS terms (AIDS OR HIV OR PLWH) and TBM-related terms (tuberculous meningitis OR TBM). Studies included in this meta-analysis evaluated the incidence of TBM among PLWH, or we were able to calculate the incidence of TBM among PLWH from the research.
The analysis revealed that the prevalence of TBM among PLWH was 13.6% (95% CI: 6.6–25.9%), with an incidence rate of 1.5 cases per 1000 persons per year. The case fatality rate was found to be 38.1% (95% CI: 24.3–54.1%). No significant publication bias was observed. Meta-regression analysis identified the proportion of females and finance situation as factors influencing the outcomes.
Conclusions
Our study highlights TBM as a prevalent opportunistic infection that targets the central nervous system in PLWH. The elevated case fatality rate is especially prominent among PLWH in impoverished regions, underscores the pressing necessity for enhanced management strategies for PLWH suffering from TBM.
Trial registration
PROSPERO; No: CRD42022338586.
Peer Review reports
Tuberculous meningitis (TBM) is a common neurological disorder in young children and people living with HIV (PLWH), and it accounts for approximately 1% of all cases of tuberculous (TB) [ 1 , 2 ]. Moreover, TBM is a fatal form of TB that kills or severely disables up to 50% of infected individuals [ 3 , 4 , 5 ]. In many regions around the world, especially in countries with TB epidemics, TB is the predominant cause of bacterial meningitis due to the protective effects of vaccination against other forms of meningitis. Moreover, the challenge of diagnosis and treatment delay is compounded by the lack of specific clinical features and the insensitivity of laboratory tests, ultimately contributing to its elevated mortality rate [ 6 ]. Critical risk factors for TBM, including age, anti-TB regimens, disease duration, and HIV infection, may contribute to the incidence and outcomes of TBM [ 7 , 8 ].
PLWHs have an increased risk of developing all forms of TB [ 9 ], coinfection with TB influences the pathogenesis of HIV and increases HIV‑1 replication in PLWH [ 10 ]. In adults, HIV-1 coinfection is the most significant risk factor for TBM. Additionally, TBM has become a severe HIV-associated CNS opportunistic infection and has increased the relative risk of death from two- to three-fold in PLWH, even with the introduction of antiretroviral therapy (ART) [ 11 ]. In the context of HIV infection, the pathological, clinical, and laboratory findings in patients with TBM are influenced in various ways, and the outcomes of TBM may be poor [ 12 ]. Although previous studies have shown that TBM is linked to a significant risk of neurological complications and death [ 13 , 14 , 15 ], and Purmohamad et al. found a high incidence of TBM-HIV coinfection [ 16 ], there is currently a lack of systematic analysis of the incidence, prevalence, and mortality rates of TBM infection in adult PLWH.
In this study, we performed a systematic review and meta-analysis to understand the global prevalence, incidence and case fatality of PLWH with TBM. Additionally, we aimed to analyse the potential risks of death associated with TBM in PLWH, providing new insights into strategies for diagnosing and treating the disease.
This study is registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration No: CRD42022338586). Furthermore, this study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [ 17 ]. The PRISMA checklist is provided in Supplement Table 1 .
Search strategy
We performed a comprehensive search in PubMed, Embase, and Web of Science from database inception to September 19th, 2023, with no limitations on the publication type. To avoid bias due to language barriers, we only searched the articles written in English. The search terms were HIV/AIDS terms (AIDS OR HIV OR PLWH) and TBM-related terms (tuberculous meningitis OR TBM). We further screened the reference lists of the selected articles to identify missing studies. A detailed description of the search strategies is provided in Supplement Table 2 .
Selection criteria
Studies included in this meta-analysis evaluated the incidence of TBM among PLWH, or provided data that allow for the calculation of the incidence of TBM among PLWH from the presented research. We excluded the following types of studies: (1) not related to HIV/AIDS; (2) research protocols or feedback reports; (3) case reports; (4) review articles; (5) children-oriented; (6) comparative study; (7) the prevalence or the incidence of TBM among PLWH could not be extracted; (8) participants less than 20. Researchers (XC and JW) removed duplicate studies using EndNote X9 software, selected preliminary search results through title and abstract, and then further determined the remaining articles by assessing full-text. Disagreements between reviewers about eligibility were resolved by discussion with TZ and YZ.
Data extraction
Two researchers (XC and JW) independently extracted and cross-checked relevant data using an Excel spreadsheet. The main information was the prevalence, incidence, and case fatality of TBM among PLWH. Additionally, we extracted other information from these articles, including authorship, year of publication, type of study, study location, sample size, mean age of participants, sex distribution, and current CD4 + T cell count from the total PLWH population and the TBM population.
Statistical analysis
Comprehensive Meta-Analysis (CMA) Version 3.0 (Biostat, Englewood, New Jersey) was adopted for quantitative analysis. Firstly, we calculated the combined event rate (ER) using the prevalence and death of TBM, and the total number of samples included. Next, the number of cases in the cohort articles was divided by the number of months of surveillance and then multiplied by 12 to calculate the number of cases per year [ 18 ]. Person-years were represented by the total number of HIV cases in each country during the study year, obtained from the UNAIDS website ( https://www.unaids.org/en/regionscountries/countries ). The rate was the number of cases per year multiplied by person per year. In addition, we conducted a random effects meta-analysis using the ER to estimate the prevalence and the case fatality of TBM among PLWH and used the rate to estimate the incidence of TBM among PLWH. Additionally, the Egger ranks correlation test was adopted to assess publication bias across studies. The I 2 and Q-tests were used to evaluate the proportion and statistical significance of heterogeneity. The threshold for statistical significance was 2-tailed p < 0.05.
Quality assessment
Two researchers (XC and JW) used the Agency of Healthcare Research and Quality (AHRQ) methodology checklist ( http://www.ncbi.nlm.nih.Gov/books/NBK35156/ ) for cross-sectional studies and independently assessed the individual studies. The checklist contained 11 items: (1) information source, (2) study criteria, (3) study period, (4) sampling, (5) interview method, (6) instrument validation, (7) exclusion criteria, (8) the measurement of confounding effects, (9) the process of dealing with missing values, (10) response rate, and (11) the use of follow-up assessment. According to this standard, studies were divided into three levels: high-quality (over 8 points), moderate-quality (4–7 points), and low-quality (0–3 points).
Meta regression
The primary outcome of our study was the prevalence and incidence of TBM among PLWH, and the secondary outcome was the case fatality of TBM among PLWH. The predefined continuous moderators were age, gender (the proportion of women), the current median/mean CD4 + T cell counts among PLWH and TBM, finance situation (Per capita gross domestic product (GDP) was obtained from the world bank website: https://data.worldbank.org.cn ), and study quality. The categorical moderators were the study period, and the study design. Restricted ML meta-regression was used to evaluate associations. The results with p < 0.05 were considered as significance-level factors.
Search results
After removing all duplicates from the 1824 records, 1821 studies were screened. Of these, 1779 records were excluded based on the title and abstract, and 42 full-text articles were assessed for eligibility. Overall, 17 eligible studies were included in the meta-analysis. A flowchart of the study selection is shown in Fig. 1 , and the details of the excluded articles are shown in Supplementary Table 3 .

The flowchart of study selection
Study characteristics
Among the 17 studies included, 9 were retrospective, and 7 were prospective. The remaining studies were a combination of retrospective and prospective designs. In addition, 12 were cross-sectional studies, and five were cohort studies. Studies included data from Africa, Europe, Asia, and South America, comprising 6561 participants. Thirteen studies reported a mean age of 35 (range, 28–39) years; the mean female proportion was 36% (range, 14–68%). The current CD4 + T cell counts was 13–158 cells/µl in PLWH and 14–142 cells/µl in TBM. Six studies were considered moderate-quality studies, whereas the remaining were high-quality (Supplementary Table 4 ). Additional characteristics of the included studies and their corresponding participants are presented in Table 1 .
Meta-analysis of prevalence, incidence and case fatality of TBM
Twelve studies reported the prevalence of TBM among PLWH. The combined ER of TBM was 13.6% (95% confidence interval [CI]: 6.6–25.9%). Significant heterogeneity was detected (Q: 520.4; I 2 : 97.9%; p < 0.001). Publication bias of the included studies had no statistical significance (intercept: -0.1, 95% CI: -114.4 to 14.3; p = 0.9) (Fig. 2 ).
Five studies reported the incidence of TBM among PLWH. The combined rate of TBM was 1.5 per 1000 per year (95% CI: 0.6–2.5 per 1000 per year). Significant heterogeneity was detected (Q: 43.9; I 2 : 90.9%; p < 0.001). Publication bias of the included studies had no statistical significance (intercept: 5.6, 95% CI: -2.5 to 13.7; p = 0.1) (Fig. 3 ).
Case fatality
Twelve studies reported on the case fatality of patients with TBM. The combined ER was 38.1% (95% CI: 24.3–54.1%), and a significant heterogeneity was detected (Q: 69.1; I 2 : 84.1%; p < 0.001). No significant publication bias was found (intercept: -1.5, 95% CI: -5.8 to 2.8; p = 0.4). The detailed results of the meta-analysis are presented in Fig. 4 .
Meta-regression
We conducted meta-regressions on all factors extracted from the studies to analyse the prevalence and case fatality among patients with TBM. While no significant results were found for prevalence, two factors produced substantial changes in outcomes: female proportion (coefficient: 0.05; p < 0.01) and finance situation (coefficient: -1.16; p = 0.02). The detailed results for the meta-regression are available in Supplementary Table 5 .
This comprehensive systematic review and meta-analysis was conducted to elucidate the prevalence incidence of TBM in PLWH and the corresponding case fatality in this population. We found that the prevalence, incidence and case fatality were approximately 13.6%, 1.5 per 1000 per year, and 38.1%, respectively, indicating that the prevalence and incidence of TBM were relatively low, while TBM-associated case fatality was high in PLWH. Moreover, the results of the meta-regression analysis suggested that the proportion of females and the finance situation may have substantial impacts on the outcomes.
PLWH are approximately 14 times more likely to develop TB, and face more than double the mortality rate during TB treatment compared to the general population [ 19 ]. PLWH are at heightened risk for TBM, particularly at more advanced stages of immunosuppression [ 20 ]. Moreover, TBM in PLWH has a poor prognosis, with no appreciable difference in survival probability based on ART timing [ 21 ]. Navarro-Flores et al. found that the prevalence of HIV was positively associated with the prevalence of TBM, identifying HIV infection as a moderator in the prevalence of TBM among hospitalized patients [ 13 ]. Moreover, a meta-analysis conducted by Stadelman et al. also suggested that the mortality of adult TBM was high and is influenced by HIV status [ 22 ]. Our study demonstrated that TMB-associated case fatality is high in PLWH, consistent with previous research on patients with TB [ 4 ].
Several risk factors influence the outcomes of CNS OIs in PLWH, including region distribution, age, sex, prophylactic history, and CD4 + T cell counts [ 23 ]. Our study analysed the related risk factors, including age, sex (the proportion of women), the current median/mean CD4 + T cell counts, and financial situation among PLWH and TBM. However, we did not find significant results for prevalence.
In addition, we found a marginal correlation effect (coefficient: -1.16; p = 0.02) between case fatality and socioeconomic status, indirectly indicating the region’s impact on case fatality. The changes in outcomes influenced by regional distribution are consistent with previous studies on TB/HIV coinfection, which showed that high morbidity and mortality rates were observed among PLWH and TB in resource-limited countries such as those in sub-Saharan Africa [ 24 ].
In our study, another factor influencing case fatality was the proportion of females, which suggests that as the proportion of females increases, so does the case fatality rate. The worldwide incidence of TB has consistently been higher in men compared with women, although the male-to-female ratio varies per region [ 25 ]. However, HIV infection significantly contributed to the epidemic rates of TB in women, leading to a 5.3-fold increase, which was notably higher compared to men. Since there are only a few studies on sex in patients with TBM, we speculate that our findings could be attributed to the increased rates of TBM in women with the onset of HIV and more susceptibility to HIV.
In certain cohorts, the mortality rate among HIV patients coinfected with TBM exceeds 50% [ 26 ]. Important contributing factors include the level of immunosuppression due to HIV and immune reconstitution inflammatory syndrome (IRIS) resulting from ART [ 27 , 28 ]. In our study, we used CD4 + T cell counts to measure the degree of immunosuppression in PLWH and found that the current CD4 + T cell counts ranged from 13 to 96 cells/µl among PLWH and 12–142 cells/µl among PLWH with TBM, suggesting that these populations are severely immunocompromised. The CD4 + T cell counts may be associated with the case fatality of PLWH and TBM in our study. However, our study did not mention other potential risk factors among PLWH and TBM, such as drug-resistant TB, specific anti-TB regimens, disease duration, and the presence of accompanying pulmonary TB or other systemic TB, which could also be critical in determining the case fatality rate.
This study had several limitations. First, the number of included studies was limited, which restricted the generalizability of the results. Second, PLWH with TBM typically experience severe immunosuppression, characterized by significantly low CD4 + T cell counts. Therefore, it may affect the generalization of our findings to all PLWH. Third, the incomplete and discrepant data in the original articles, coupled with a dearth of detailed information from individual studies, resulted in inadequate subgroup analyses. For example, age and CD4 + T cell counts showed no remarkable difference in the prevalence and case fatality. This lack of significance could be attributed to several factors: (1) in all studies, the mean age was approximately 30 years, which is consistent with the peak age at onset of TB; and (2) since the population in our study was PLWH and TBM, the CD4 + T cell counts in each study were relatively low, with the highest not exceeding 150 cells/µl, leading to low statistical power in this subgroup analysis. In addition, the regional distribution is limited owing to few relevant studies. Thus, our findings of regional differences do not reflect the global distribution and lack validity and generalizability. For example, the outcomes of the regional distribution in the population suggested that Europe has the most significant number of studies, the prevalence of TBM in PLWH is generally high (up to 13%), and the prevalence in Africa and South America is nearly 10%. In contrast, the prevalence in Asia is as high as 55.3% (Supplement Fig. 1 ); the case fatality in Africa was the highest (up to 60%); this disparity may be linked to limited access to treatment, poverty, gender inequality, and societal stigma towards HIV in Africa [ 29 , 30 ]. When compared to other regions, this situation is more widespread in Africa [ 31 , 32 ] (Supplement Fig. 2 ). Fourth, the heterogeneity in this meta-analysis was high (Q: 72.081; I²: 81.97%; p < 0.001), likely due to significant differences in study design, including variations in sample sizes, methodologies, and population characteristics. This heterogeneity may contribute to limited generalizability and potential biases in our study.

Meta-analysis of prevalence of TBM among PLWH

Meta-analysis of incidence of TBM among PLWH

Meta-analysis of case fatality of TBM among PLWH
In conclusion, this systematic review and meta-analysis provided a comprehensive epidemiological landscape of TBM in PLWH exhibiting low prevalence and incidence rates alongside high case fatality rates, particularly those from economically disadvantaged areas. Additionally, meta-regressions were performed to investigate the pertinent risk factors influencing TBM prevalence and case fatality rates among PLWH. Notably, the financial situation and the proportion of females were identified as significant factors influencing these outcomes. This study underscores the critical necessity for immediate public health interventions to enhance patient care for individuals affected by PLWH and TBM.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- Tuberculous meningitis
Tuberculous
People living with HIV
Central nervous system
Opportunistic infections
Antiretroviral therapy
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Comprehensive Meta-Analysis
Agency of Healthcare Research and Quality
Gross domestic product
Progressive multifocal leukoencephalopathy
Immune reconstitution inflammatory syndrome
Jain S, Tobin D, Tucker E, Venketaraman V, Ordonez A, Jayashankar L, Siddiqi O, Hammoud D, Prasadarao N, Sandor M, et al. Tuberculous meningitis: a roadmap for advancing basic and translational research. Nat Immunol. 2018;19(6):521–5.
Article CAS PubMed PubMed Central Google Scholar
Marais S, Thwaites G, Schoeman J, Török M, Misra U, Prasad K, Donald P, Wilkinson R, Marais B. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis. 2010;10(11):803–12.
Article PubMed Google Scholar
Wilkinson R, Rohlwink U, Misra U, van Crevel R, Mai N, Dooley K, Caws M, Figaji A, Savic R, Solomons R, et al. Tuberculous meningitis. Nat Reviews Neurol. 2017;13(10):581–98.
Article Google Scholar
Graham S, Donald P. Death and disability: the outcomes of tuberculous meningitis. Lancet Infect Dis. 2014;14(10):902–4.
Dian S, Rahmadi R, van Laarhoven A, Ganiem A, van Crevel R. Predicting Mortality of Tuberculous Meningitis. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2018;67(12):1954–5.
Thwaites G, van Toorn R, Schoeman J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 2013;12(10):999–1010.
Article CAS PubMed Google Scholar
Huynh J, Donovan J, Phu N, Nghia H, Thuong N, Thwaites G. Tuberculous meningitis: progress and remaining questions. Lancet Neurol. 2022;21(5):450–64.
Tenforde M, Mokomane M, Leeme T, Tlhako N, Tsholo K, Chebani T, Stephenson A, Hutton J, Mitchell H, Patel R, et al. Mortality in adult patients with culture-positive and culture-negative meningitis in the Botswana national meningitis survey: a prevalent cohort study. Lancet Infect Dis. 2019;19(7):740–9.
Article PubMed PubMed Central Google Scholar
Berenguer J, Moreno S, Laguna F, Vicente T, Adrados M, Ortega A, González-LaHoz J, Bouza E. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326(10):668–72.
Bell L, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90.
Heemskerk A, Bang N, Mai N, Chau T, Phu N, Loc P, Chau N, Hien T, Dung N, Lan N, et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374(2):124–34.
Garg R, Sinha M. Tuberculous meningitis in patients infected with human immunodeficiency virus. J Neurol. 2011;258(1):3–13.
Navarro-Flores A, Fernandez-Chinguel JE, Pacheco-Barrios N, Soriano-Moreno DR, Pacheco-Barrios K. Global morbidity and mortality of central nervous system tuberculosis: a systematic review and meta-analysis. J Neurol. 2022;269(7):3482–94.
Nataprawira HM, Gafar F, Risan NA, Wulandari DA, Sudarwati S, Marais BJ, Stevens J, Alffenaar J-WC, Ruslami R. Treatment outcomes of Childhood Tuberculous meningitis in a real-world retrospective cohort, Bandung, Indonesia. Emerg Infect Dis. 2022;28(3):660–71.
Merkler AE, Reynolds AS, Gialdini G, Morris NA, Murthy SB, Thakur K, Kamel H. Neurological complications after tuberculous meningitis in a multi-state cohort in the United States. J Neurol Sci. 2017;375:460–3.
Purmohamad A, Azimi T, Nasiri MJ, Goudarzi M, Zangiabadian M, Sedighian H, Fooladi AAI. HIV-Tuberculous meningitis Co-infection: a systematic review and Meta-analysis. Curr Pharm Biotechnol. 2021;22(7):960–8.
Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Shrestha DB, Budhathoki P, Gurung B, Subedi S, Aryal S, Basukala A, Aryal B, Adhikari A, Poudel A, Yadav GK et al. Epidemiology of dengue in SAARC territory: a systematic review and meta-analysis. Parasite Vector 2022, 15(1).
WHO. Global tuberculosis report 2023. 2023.
Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep. 2009;6(3):139–45.
Lawn S, Wood R. Poor prognosis of HIV-associated tuberculous meningitis regardless of the timing of antiretroviral therapy. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2011;52(11):1384–7.
Stadelman AM, Ellis J, Samuels THA, Mutengesa E, Dobbin J, Ssebambulidde K, Rutakingirwa MK, Tugume L, Boulware DR, Grint D, et al. Treatment outcomes in adult tuberculous meningitis: a systematic review and Meta-analysis. Open Forum Infect Dis. 2020;7(8):ofaa257.
Agnihotri S. Central Nervous System opportunistic infections. Semin Neurol. 2019;39(3):383–90.
Osman M, Welte A, Dunbar R, Brown R, Hoddinott G, Hesseling A, Marx F. Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Diseases: IJID : Official Publication Int Soc Infect Dis. 2019;85:57–63.
Google Scholar
WHO. Global tuberculosis report. 2021.
Thao LTP, Heemskerk AD, Geskus RB, Mai NTH, Ha DTM, Chau TTH, Phu NH, Chau NVV, Caws M, Lan NH, et al. Prognostic models for 9-Month Mortality in Tuberculous Meningitis. Clin Infect Diseases: Official Publication Infect Dis Soc Am. 2018;66(4):523–32.
Article CAS Google Scholar
Marais S, Lai RPJ, Wilkinson KA, Meintjes G, O’Garra A, Wilkinson RJ. Inflammasome Activation Underlying Central Nervous System Deterioration in HIV-Associated Tuberculosis. J Infect Dis. 2017;215(5):677–86.
CAS PubMed Google Scholar
Thwaites GE, N DB, N HD, H TQ, D TTO, N TCT, N QH, N TT, N NH, N TNL, et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with tuberculous meningitis. J Infect Dis. 2005;192(12):2134–41.
Harrison A, Colvin CJ, Kuo C, Swartz A, Lurie M. Sustained high HIV incidence in Young Women in Southern Africa: social, behavioral, and structural factors and emerging intervention approaches. Curr HIV/AIDS Rep. 2015;12(2):207–15.
Delva W, Abdool Karim Q. The HIV epidemic in Southern Africa - is an AIDS-free generation possible? Curr HIV/AIDS Rep 2014, 11(2).
Shah G, Ewetola R, Etheredge G, Maluantesa L, Waterfield K, Engetele E, Kilundu A. Risk factors for TB/HIV coinfection and consequences for patient outcomes: evidence from 241 clinics in the Democratic Republic of Congo. Int J Environ Res Public Health 2021, 18(10).
Dong K, Thabethe Z, Hurtado R, Sibaya T, Dlwati H, Walker B, Wilson D. Challenges to the success of HIV and Tuberculosis care and treatment in the public health sector in South Africa. J Infect Dis 2007:S491–496.
Download references
Acknowledgements
Not applicable.
This research was funded by the Beijing Natural Science Foundation (Z220018, 7222095); the Capital’s Funds for Health Improvement and Research (2022-1-1151), the National Key R&D Program of China (2023YFE0116000, 2023YFC2308300), the National Natural Science Foundation of China (81571178, 81873761, 82072271); the Climbing the Peak (Deng feng) Talent Training Program of the Beijing Hospitals Authority (DFL20191701); the Beijing Health Technologies Promotion Program (BHTPP2020); and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089); Beijing research center for respiratory infectious diseases project (BJRID2024-001).
Author information
Xue Chen, Jiaqi Wei, Mei Zhang these authors contributed equally to this work.
Authors and Affiliations
Clinical and Research Center for Infectious Diseases, Beijing Youan Hospital, Beijing Key Laboratory for HIV/AIDS Research, Capital Medical University, Beijing, 100069, China
Xue Chen, Jiaqi Wei, Mei Zhang, Bin Su, Meixin Ren & Tong Zhang
Beijing Youan Hospital, Beijing Institute of Hepatology, Capital Medical University, Beijing, 100069, China
Department of Respiratory and Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, Beijing, 100069, China
Miaotian Cai & Yulin Zhang
Beijing Research Center for Respiratory Infectious Diseases, Beijing, 100069, China
Yulin Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: XC, JW; methodology and software; analysis: JW and MZ; writing—original draft preparation: XC, JW; writing—review and editing: XC, MR, BS and MC; supervision and funding acquisition: YZ, TZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Yulin Zhang or Tong Zhang .
Ethics declarations
Ethics approval consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chen, X., Wei, J., Zhang, M. et al. Prevalence, incidence, and case fatality of tuberculous meningitis in adults living with HIV: a systematic review and meta-analysis. BMC Public Health 24 , 2145 (2024). https://doi.org/10.1186/s12889-024-19683-4
Download citation
Received : 12 March 2024
Accepted : 02 August 2024
Published : 07 August 2024
DOI : https://doi.org/10.1186/s12889-024-19683-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Epidemiology
- Meta-analysis
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]

NYU GPH Journal of Public Health Disasters
A student-led journal driven by the latest research in public health disaster preparedness and management.
Tag: Health Systems
Tech outage causes worldwide disruptions.

On the morning of Friday, July 19th, CrowdStrike customers experienced a major digital blackout as outages hit banks, hospitals, airports, and other services worldwide. Businesses scrambled to mitigate the effects of the sudden disruption, implement plans, rely on backup systems, and maintain operations to provide customer service.
Susan Nagel’s Case Study on Tuberculosis in Alabama
From 2014-2016, an unprecedented tuberculosis outbreak occurred in Marion County in Alabama, resulting in an incident rate worse than experienced in many low and middle-income countries. Susan Nagel’s presentation discusses why the outbreak occurred, its effects, and the management of the event. The case study concludes that structural racism in healthcare settings must be addressed to prevent similar outbreaks from occurring in the future.
Improving Consistency in Health System Resilience Research
The concept of health system resilience is one that evokes images of communities and healthcare infrastructures and systems weathering a devastating event and working together to rebuild and emerge stronger. This idea is beginning to gain attention, as the subject of empirical studies more recently but has mostly been explored only conceptually in the past.
ORIGINAL RESEARCH article
Spatiotemporal analysis of tuberculosis in the hunan province, china, 2014–2022.

- 1 Chinese Center for Disease Control and Prevention, Beijing, China
- 2 Department of Science and Education, Hunan Chest Hospital, Changsha, China
- 3 Department of Tuberculosis Control and Prevention, Hunan Chest Hospital, Changsha, China
- 4 Hunan Chest Hospital, Changsha, China
Background: Pulmonary tuberculosis (PTB) is a major infectious disease that threatens human health. China is a high tuberculosis-burden country and the Hunan Province has a high tuberculosis notification rate. However, no comprehensive analysis has been conducted on the spatiotemporal distribution of PTB in the Hunan Province. Therefore, this study investigated the spatiotemporal distribution of PTB in the Hunan Province to enable targeted control policies for tuberculosis.
Methods: We obtained data about cases of PTB in the Hunan Province notified from January 2014 to December 2022 from the China Information System for Disease Control and Prevention. Time-series analysis was conducted to analyze the trends in PTB case notifications. Spatial autocorrelation analysis was conducted to detect the spatial distribution characteristics of PTB at a county level in Hunan Province. Space-time scan analysis was conducted to confirm specific times and locations of PTB clustering.
Results: A total of 472,826 new cases of PTB were notified in the Hunan Province during the 9-year study period. The mean PTB notification rate showed a gradual, fluctuating downward trend over time. The number of PTB notifications per month showed significant seasonal variation, with an annual peak in notifications in January or March, followed by a fluctuating decline after March, reaching a trough in November or December. Moran’s I index of spatial autocorrelation revealed that the notification rate of PTB by county ranged from 0.117 to 0.317 during the study period, indicating spatial clustering. The hotspot areas of PTB were mainly concentrated in the Xiangxi Autonomous Prefecture, Zhangjiajie City, and Hengyang City. The most likely clustering region was identified in the central-southern part of the province, and a secondary clustering region was identified in the northwest part of the province.
Conclusion: This study identified the temporal trend and spatial distribution pattern of tuberculosis in the Hunan Province. PTB clustered mainly in the central-southern and northwestern regions of the province. Disease control programs should focus on strengthening tuberculosis control in these regions.
1 Introduction
Pulmonary tuberculosis (PTB) is a major infectious disease that seriously threatens human health. According to the WHO Global Tuberculosis Report 2023 ( 1 ), an estimated 10.6 million new cases of PTB occurred globally in 2022, with 1.3 million deaths. PTB remains an important public health problem. After decades of efforts, the incidence of tuberculosis has gradually declined in China. However, the incidence of tuberculosis within the country varies by region, with some areas still experiencing major outbreaks ( 2 , 3 ). The Hunan Province, located in central-southern China, has reported high numbers of cases and PTB notification rates. In 2022, 43,976 new PTB cases were notified, with a notification rate of 66.40/100,000, which was higher than the national average. Therefore, more effective control strategies are urgently needed to curb the spread of tuberculosis in the Hunan Province.
Spatiotemporal analysis methods have been used extensively to investigate the distribution and variation patterns of PTB in recent years. Studies have been conducted in several countries, including Russia ( 4 ), Brazil ( 5 , 6 ), Uganda ( 7 ), and Peru ( 8 ). In China, researchers such as Zhang et al. ( 9 ) have studied the spatiotemporal distribution characteristics of tuberculosis at a provincial level, whereas others such as Liu et al. ( 3 ) have conducted similar analyses at a prefecture level. Studies of the spatiotemporal distribution of tuberculosis have been conducted in Beijing ( 10 ), Zhejiang ( 11 , 12 ), Chongqing ( 13 ), and Qinghai ( 14 ) Provinces. In the Hunan Province, Alene et al. ( 15 ) examined spatiotemporal distribution patterns, and Zheng et al. ( 16 ) investigated the spatial clustering and hotspot areas of smear-positive PTB notifications in the Hunan Province in 2012 and 2013. However, to our knowledge, no comprehensive analysis has been conducted on the spatiotemporal distribution of PTB in the whole of the Hunan Province. The current spatiotemporal distribution and trends in the spatiotemporal distribution of PTB in the Hunan Province remain unclear and warrant further investigation.
This study aimed to analyze the temporal distribution characteristics of tuberculosis in the Hunan Province, examining the clustering of PTB notifications at a county level to identify hotspot and cold-spot areas of incidence. Through spatiotemporal scanning analysis, this study aimed to pinpoint specific locations of tuberculosis incidence clustering and assess the magnitude of disease risk in these clusters. By identifying the key regions of PTB incidence in the Hunan Province, these results provide a source of reference for developing targeted tuberculosis control strategies.
2 Materials and methods
2.1 overview of the study area.
The Hunan Province is in the central-southern region of China ( Figure 1 ), which covers an area of 211,800 square kilometers. Its terrain is characterized by mountains and hills, with mountainous areas covering 51.2%, hills and plateaus covering 29.3%, plains covering 13.1%, and water surfaces covering 6.4% of the total provincial area. The number of permanent residents was 66.04 million in 2022. The Hunan Province includes 14 prefecture-level administrative divisions, with 122 county-level administrative divisions ( 17 ).
Figure 1 . Location of the Hunan Province.
2.2 Data collection
Data on PTB notifications for each county in the Hunan Province from January 2014 to December 2022 were download from the China Information System for Disease Control and Prevention (CISDCP). The dataset included the number of PTB notifications for each county by month. PTB notifications included both laboratory-confirmed and clinically diagnosed cases. From January 2014 to April 2018, the diagnostic criteria for tuberculosis were based on the National Health Commission of the People’s Republic of China WS 288-2008, and from May 2018 to December 2022, the diagnostic criteria for tuberculosis were based on the National Health Commission of the People’s Republic of China WS 288-2017 ( 18 ). Population data for each county in the Hunan Province from 2014 to 2022 were obtained from the Hunan Statistical Yearbook (2015–2023). To calculate the mean PTB notification rate, we summed the number of cases notified by county for the 9-year period and divided them by the total population of each county for the same period.
The overall PTB notification rate and the notification rate of laboratory-confirmed cases of PTB were used as indicators of the effectiveness of control programs. The overall PTB notification rate included laboratory-confirmed cases, laboratory-negative cases, and cases with no laboratory results. According to the 2019 notification on the adjustment of the classification of infectious disease reports for PTB ( 19 ), laboratory-confirmed cases of PTB include smear-positive cases; smear-negative, culture-positive cases; and cases testing positive on nucleic acid amplification tests (NAATs). Since January 1, 2017, cases of rifampicin-resistant tuberculosis have been recorded in CISDCP ( 20 ). Laboratory-negative PTB refers to patients with PTB whose sputum smears and cultures are both negative, and NAAT results are negative or unavailable. PTB with no laboratory results refers to patients with tuberculosis symptoms or tuberculous pleurisy, but no sputum smear, culture, or NAAT results.
2.3 Statistical analysis
2.3.1 time-series analysis.
In the time-series analysis, we analyzed the number of notified PTB cases in the Hunan Province from January 2014 to December 2022 (108 months). Using time as the horizontal axis and the number of notified PTB cases per month as the vertical axis, we plotted a curve showing the monthly PTB cases in the Hunan Province over time. The tuberculosis incidence was analyzed by observing the trend in the curve. The analyses were conducted using Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, United States).
2.3.2 Spatial autocorrelation analysis
Spatial autocorrelation analysis was conducted to investigate whether the observed values at a particular location in a spatial area were correlated with similar observed values in neighboring areas. The analysis included global and local spatial autocorrelation. Global spatial autocorrelation can be classified into positive spatial autocorrelation, negative spatial autocorrelation, and spatial randomness (i.e., random distribution) based on the spatial distribution characteristics. Spatial autocorrelation analysis quantifies the type of spatial data correlation, explores clustering, and examines the process through which spatial features change over time, thereby identifying risk factors for disease in the study region. For the global spatial autocorrelation analysis, we used Moran’s I ( 21 ), which is widely applied as a statistical index in spatial epidemiology ( 11 , 13 ). The Moran’s I index can range between −1 and 1. A positive Moran’s I value closer to 1 indicates higher clustering, whereas a negative value closer to −1 suggests a more dispersed distribution. A value of zero indicates a random distribution. We used this method to confirm the spatial distribution characteristics of the PTB notification rate at a county level in Hunan Province from 2014 to 2022. The Z-score was calculated to assess the significance of the Moran’s I estimate. If Moran’s I is greater than 0 and the Z-score is greater than or equal to 1.96, the distribution of disease is assumed to be spatially clustered and statistically aggregated ( 22 ).
Local spatial autocorrelation analysis is used to describe spatial correlation patterns, locate specific clustering areas, and investigate local spatial instability, thus revealing spatial heterogeneity among data. In this study, the local Gi * statistic was calculated to assess local spatial autocorrelation ( 23 ) and to identify hotspot and cold-spot areas of PTB notifications. A significant positive value, such as Gi* ≥ 1.96, indicates that high values in the locality are clustered more than those in other areas. Conversely, a significant negative value, such as Gi* ≤ −1.96, indicates that low values in the locality are clustered less than those in other areas. These analyses were conducted using ArcGIS 10.2 software (ESRI Inc., Redlands, CA, United States).
2.3.3 Space-time scan statistic
The space-time scan statistic was proposed by Kulldorff ( 24 ) in 1995 to observe and infer the spatiotemporal clustering of diseases. It can detect abnormal changes in the number of occurrences of a specific event (disease) within a spatiotemporal range and test whether these changes are due to random variation. That is, it investigates whether disease clustering exists within the study region, the exact location of the clustering, and the magnitude of clustering risk, and tests whether this clustering has statistical significance. Currently, it is widely used in tuberculosis research ( 3 , 11 , 13 ).
In this study, the space-time scan statistic model used a Poisson model and focused on areas with high PTB notification rates. The space-time scan window is a cylinder in which both spatial and temporal dimensions are constantly changing, corresponding to changes in the radius and height of the cylinder base. Simultaneously, the center of the circle at the base of the cylinder moves between the center points of each spatial unit. Each time the center, radius, and height of the cylindrical window change, a log-likelihood ratio (LLR) is calculated to compare the risk inside and outside the window. The space-time scan uses the Poisson model for every position and scale of the space-time scan window. The null hypothesis assumes that the spatial distribution of the disease is completely random, whereas the alternative hypothesis suggests an increased risk of disease occurrence inside the scan window relative to outside the scan window. For each scan window, the LLR test statistic is calculated based on the disease occurrences inside and outside the window to compare the risk. The window with the maximum LLR is considered the most likely cluster, known as the primary cluster, whereas other windows displaying statistically significant LLR values are defined as secondary clusters ( 25 ). These analyses were conducted using SaTScan version 9.5 (Kulldorff, Boston, MA, United States).
2.4 Ethical review
This study was approved by the Medical Ethics Committee of Hunan Chest Hospital. All personal information and privacy protection were carried out in accordance with the ethical requirements. The requirement for informed consent did not apply because the notification data were aggregated, and no individual-level data were analyzed.
3.1 Overview of PTB in the Hunan Province
From 2014 to 2022, 472,826 cases of PTB were notified in the Hunan Province; of them, 205,176 cases were laboratory-confirmed, 243,571 cases were laboratory-negative, and 24,079 cases did not have information available on microbiological status. The percentage of laboratory-confirmed PTB increased from 38.43% in 2014 to 57.74% in 2022, whereas the percentage of cases with negative results decreased from 55.49% in 2014 to 38.56% in 2022 ( Table 1 ). The mean annual PTB notification rate in the Hunan Province between 2014 and 2022 was 77.55/100,000. The mean annual PTB notification rate in the province decreased from 85.88/100,000 in 2014 to 66.40/100,000 in 2022, showing a gradual downward trend with fluctuations. The annual notification rate of laboratory-confirmed PTB increased from 33.00/100,000 in 2014 to 38.34/100,000 in 2022, showing a fluctuating gradual upward trend ( Figure 2 ). In contrast, the notification rate of laboratory-negative PTB decreased from 47.65/100,000 in 2014 to 25.60/100,000 in 2022, showing a downward trend ( Figure 2 ).
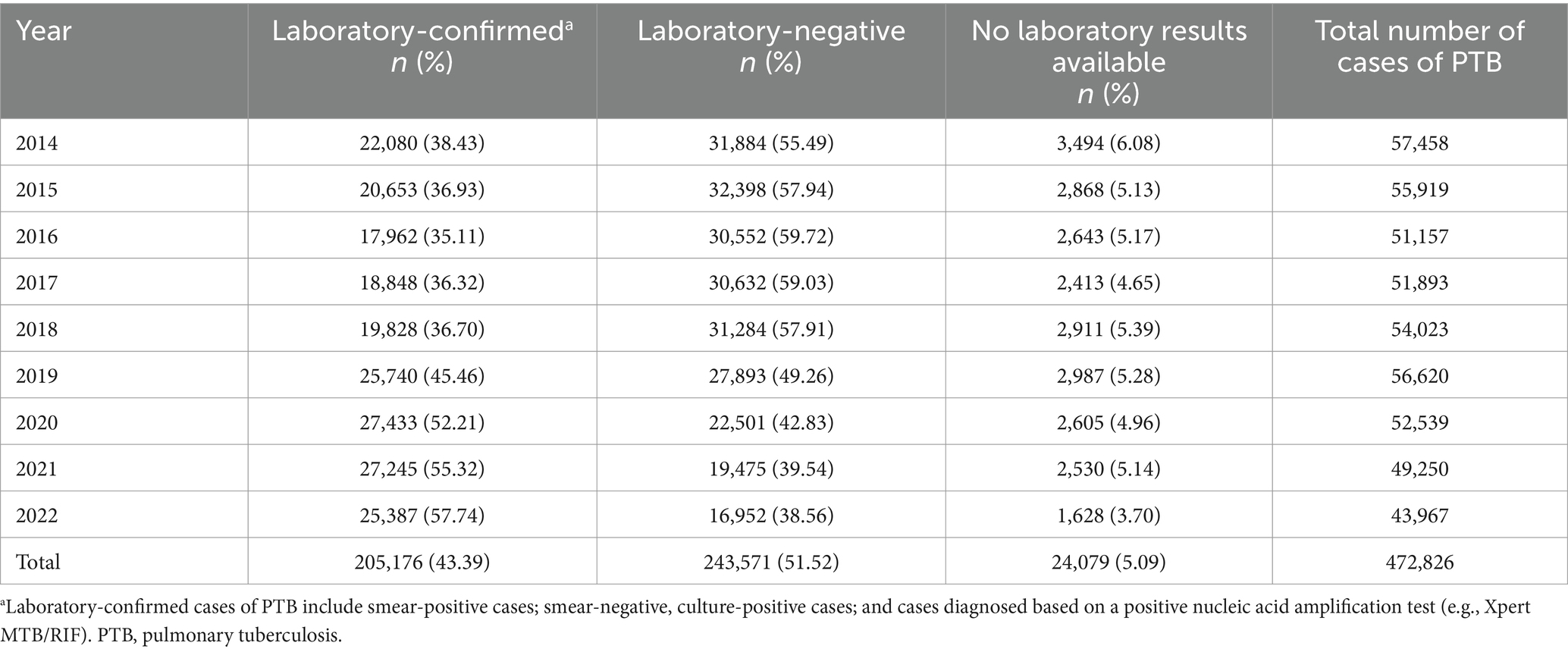
Table 1 . Laboratory confirmation of cases of PTB notified in the Hunan Province, China from 2014 to 2022.
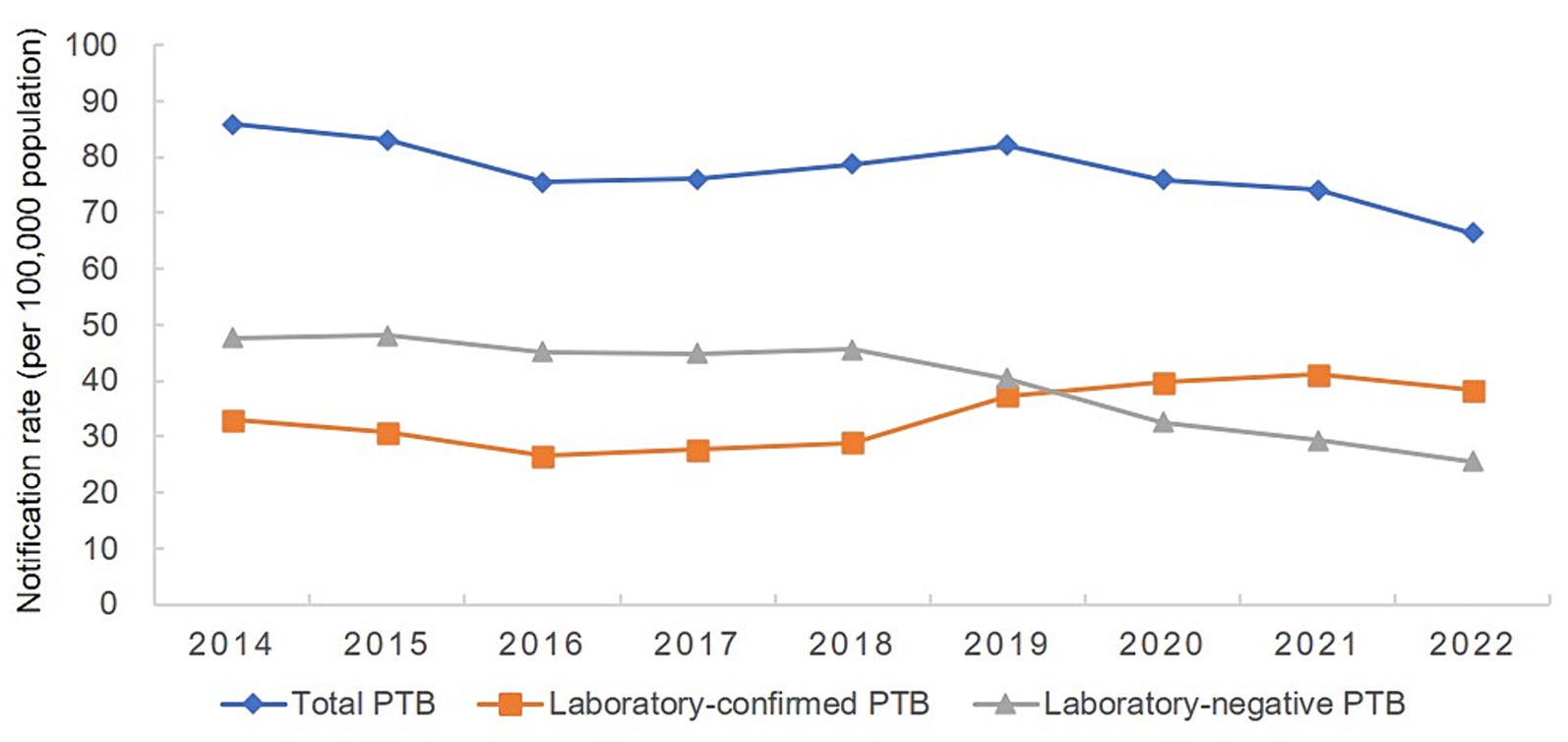
Figure 2 . Annual pulmonary tuberculosis notification rate in the Hunan Province, China from 2014 to 2022.
3.2 Temporal trends in PTB case notifications
The number of PTB notifications per month showed significant seasonal variation from 2014 to 2019 ( Figure 3 ). The number of notifications peaked in January or March every year, followed by a fluctuating decline after March, and reached a trough in November or December. However, this pattern was disrupted from 2020 to 2022, with peaks occurring in June, April, and July, in 2020, 2021, and 2022, respectively, although the troughs still occurred in December each year.
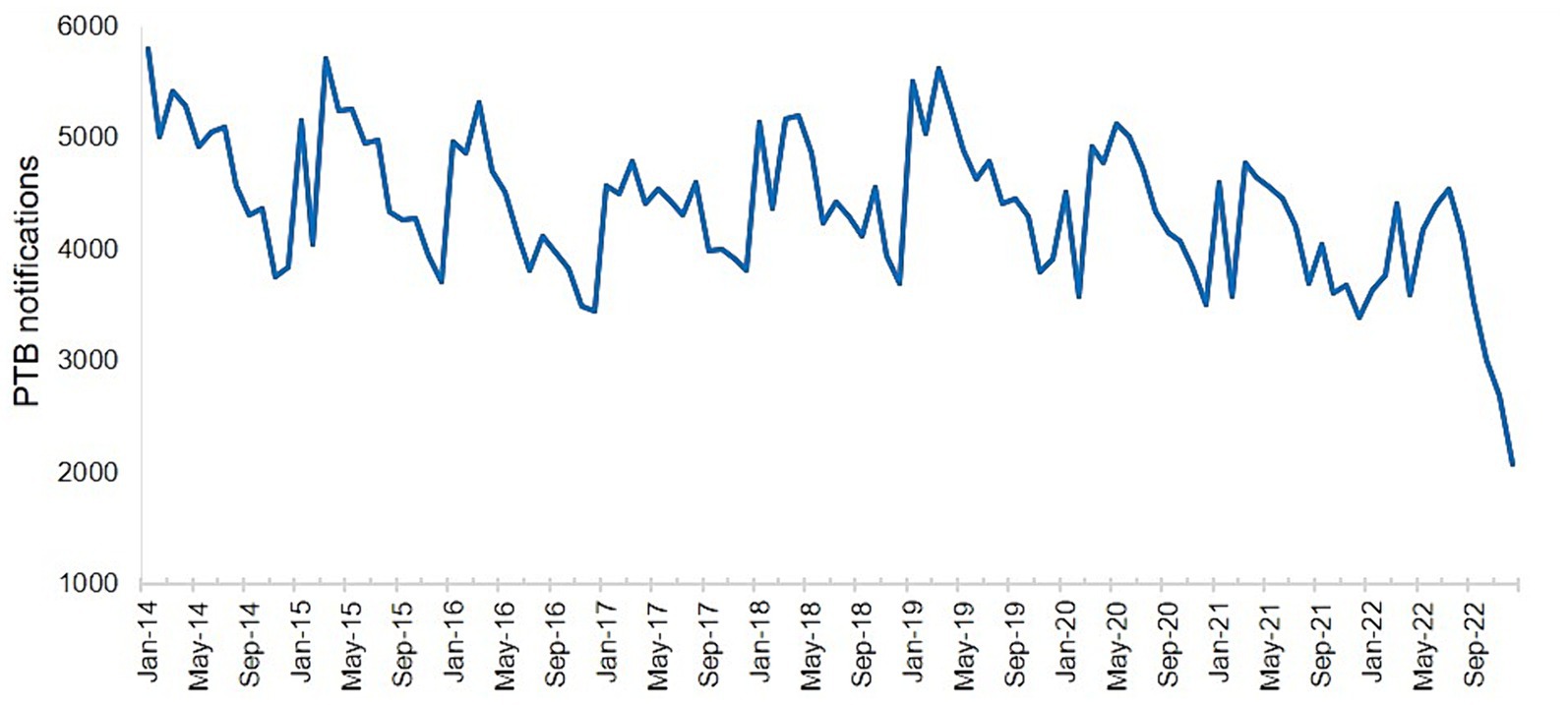
Figure 3 . Number of notified cases of pulmonary tuberculosis in the Hunan Province by month from January 2014 to December 2022.
3.3 Spatial distribution of PTB case notifications
The PTB notification rate in the Hunan Province between 2014 and 2022 varied by county and district ( Figure 4 ). Over the 9-year period, the counties with the highest mean PTB notification rates were the Hengdong, Sangzhi, Cili, Hengshan, and Fenghuang Counties. The global spatial autocorrelation analysis results showed that Moran’s I of the annual PTB notification rate at a county level ranged from 0.117 to 0.317 between 2014 and 2022 ( p < 0.05) ( Table 2 ), indicating that the occurrence of PTB exhibited a positive global spatial autocorrelation and was clustered.
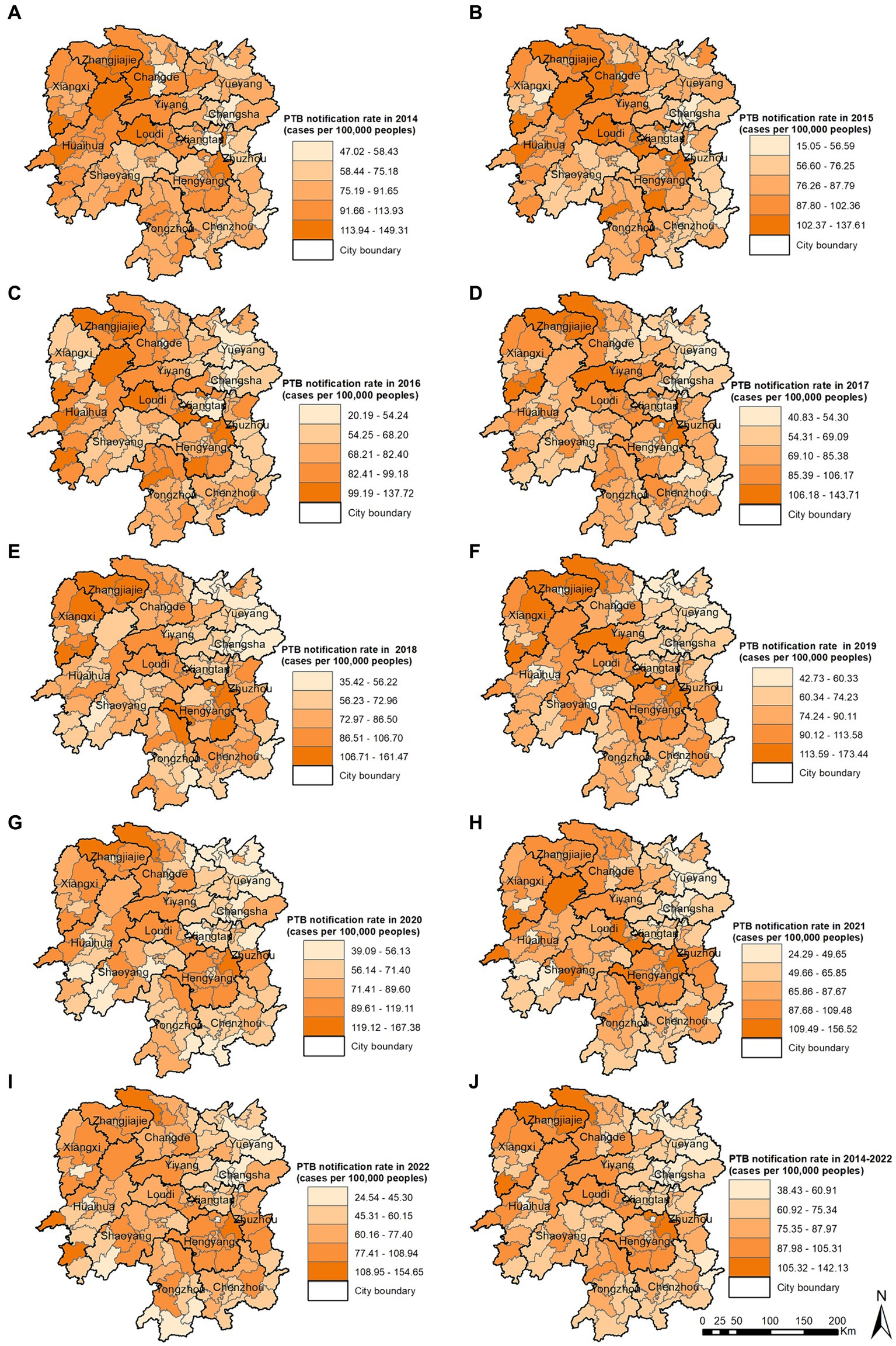
Figure 4 . Pulmonary tuberculosis notification rate in 2014 (A) , 2015 (B) , 2016 (C) , 2017 (D) , 2018 (E) , 2019 (F) , 2020 (G) , 2021 (H) , 2022 (I) , 2014–2022 (J) .
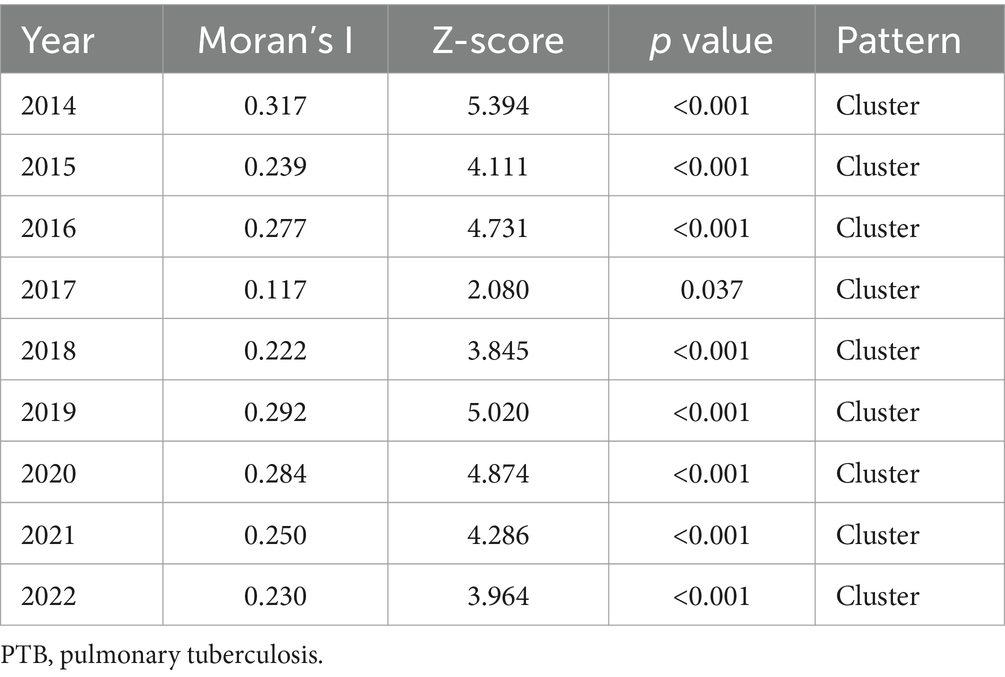
Table 2 . Global spatial autocorrelation analysis of the annual PTB notification rate in the Hunan Province, China from 2014 to 2022.
The local spatial autocorrelation analysis results revealed that the hotspot and cold-spot areas of disease incidence in the Hunan Province changed annually from 2014 to 2022. The hotspot areas were mainly concentrated in the Xiangxi Autonomous Prefecture, Zhangjiajie City, and Hengyang City, and in some adjacent areas such as the Yuanling County in Huaihua City, Shimen County in Changde City, Xinhua County in Loudi City, Anhua County in Yiyang City, Qiyang City in Yongzhou City, and You County in Zhuzhou City. From 2018 to 2022, the hotspot areas in the Xiangxi Autonomous Prefecture gradually decreased annually and disappeared in 2021 and 2022; however, hotspot areas gradually formed in the Hengyang City from 2018 to 2022. The cold-spot areas of disease incidence gradually expanded from the urban zones of Changsha, Zhuzhou, and Xiangtan in 2014 to the entire cities of Changsha, Zhuzhou, and Xiangtan, and some areas of Yueyang, by 2022 ( Figures 5A – I ). Comparison of the mean PTB notification rate and the clustering of laboratory-confirmed cases of PTB over the 9-year study period revealed patterns in hotspot and cold-spot areas. However, the hotspot areas of laboratory-confirmed cases of PTB also included the Jishou City and Fenghuang County in the Xiangxi Autonomous Prefecture, and the Mayang Miao Autonomous County and Chenxi County in Huaihua City ( Figures 5J , K ).
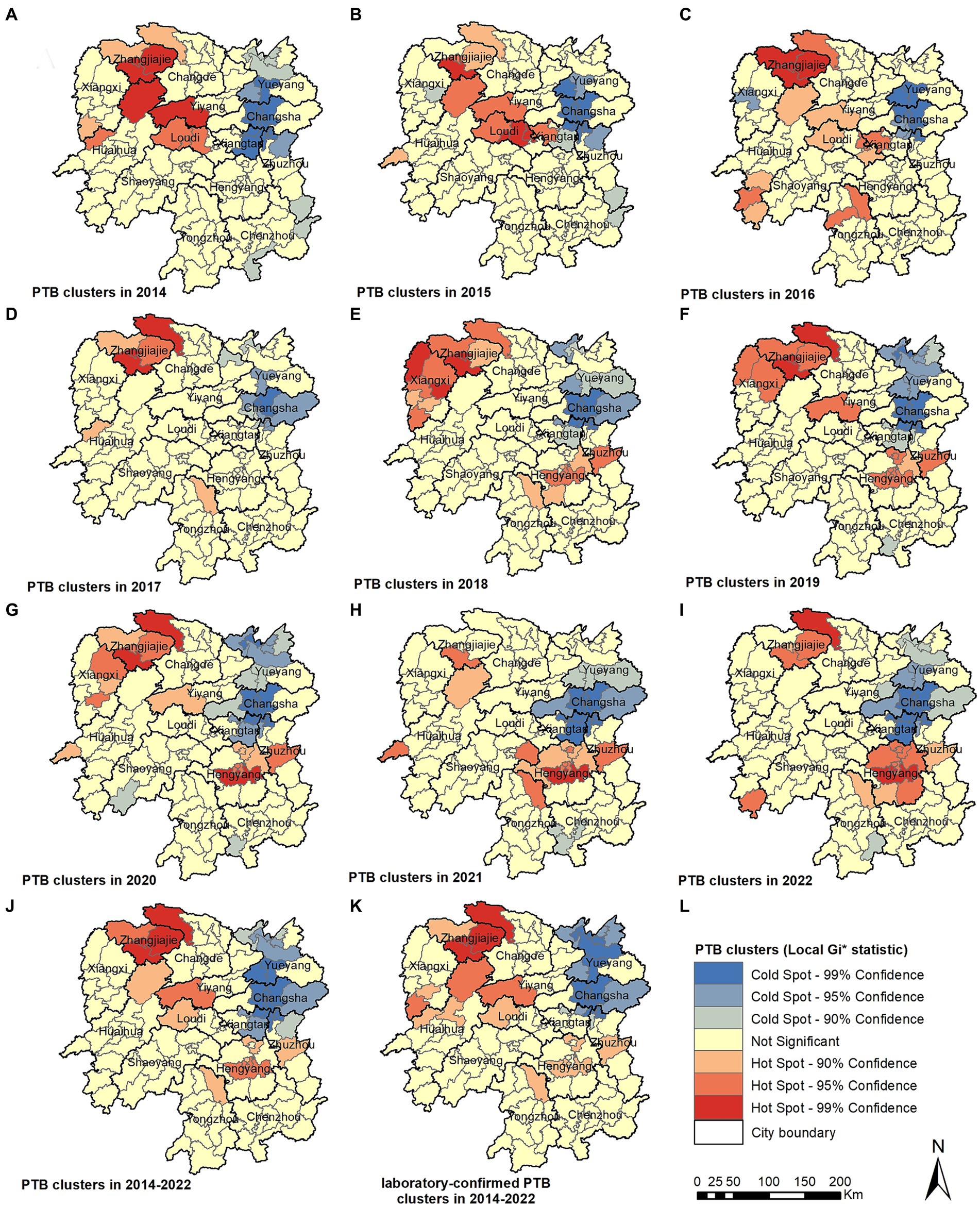
Figure 5 . Pulmonary tuberculosis clusters in 2014 (A) , 2015 (B) , 2016 (C) , 2017 (D) , 2018 (E) , 2018 (F) , 2020 (G) , 2021 (H) , 2022 (I) , 2014–2022 (J) , laboratory-confirmed PTB clusters in 2014–2022 (K) .
3.4 Spatiotemporal clustering analysis using SaTScan
The spatiotemporal scanning analysis results showed two distinct clusters of PTB incidence in the Hunan Province between 2014 and 2022. The most likely cluster was in the central-southern part of the Hunan Province, including the entire area of the Hengyang City, Anren County in Chenzhou City, Chaling County and You County in Zhuzhou City, Shuangfeng County in Loudi City, and Qiyang City in Yongzhou City, comprising 17 counties and districts. The clustering period was from March 1, 2018, to August 31, 2022, during which 47,338 cases of PTB were confirmed. The PTB notification rate within the clustered area was 1.36 times higher than that in areas outside the cluster. The secondary cluster was situated in the northwest region of the Hunan Province, encompassing the entire Xiangxi Autonomous Prefecture, Zhangjiajie City, eight county-level administrative regions in Huaihua City, Taoyuan County and Shimen County in Changde City, Anhua County in Yiyang City, and Xinhua County in Loudi City, comprising 25 counties and districts. The clustering period was from January 1, 2014, to May 31, 2018, during which 50,877 cases of PTB were notified. The PTB notification rate within the clustered area was 1.3 times higher than that in areas outside the cluster ( Table 3 ; Figure 6 ).
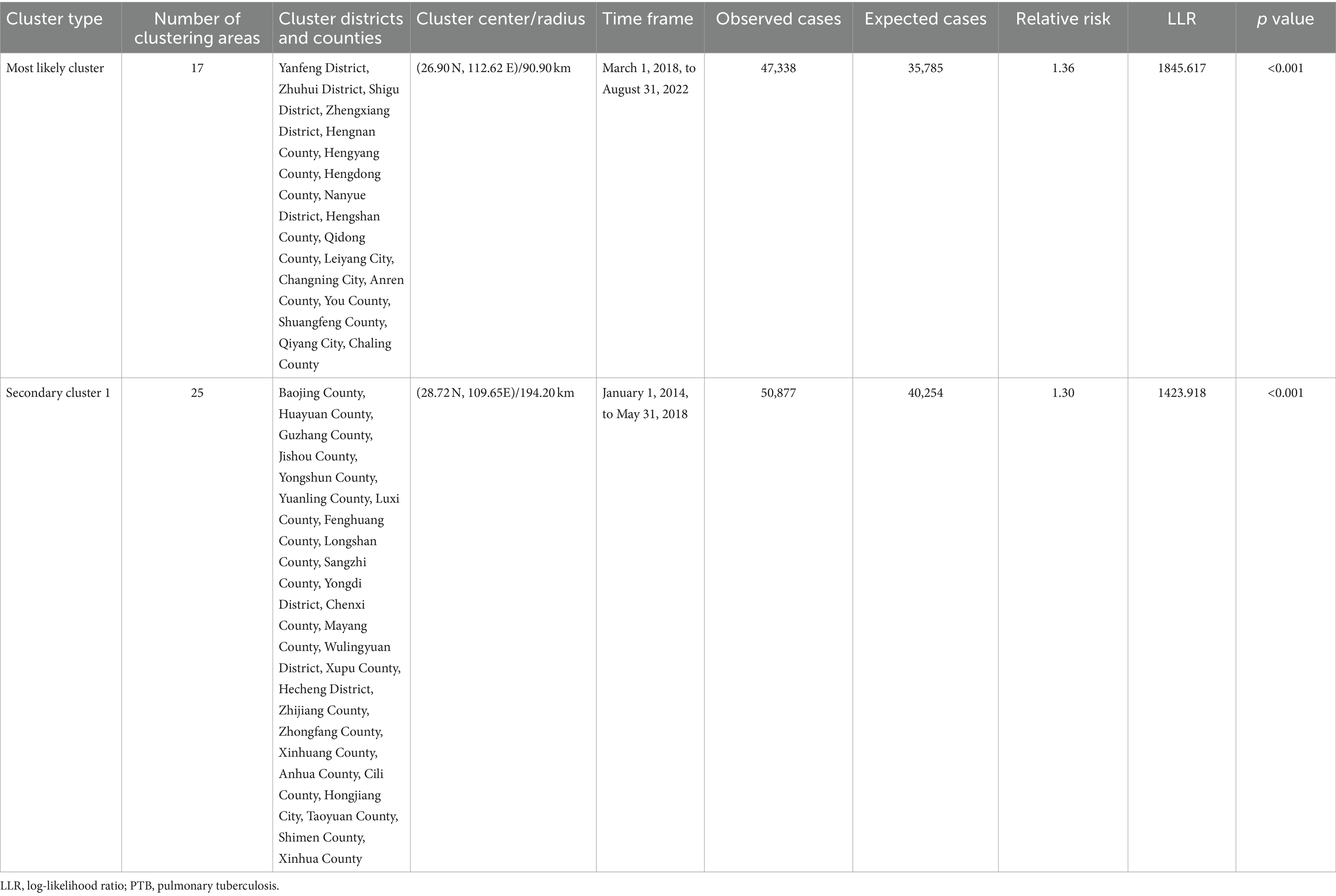
Table 3 . Space-time clustering of cases of PTB in the Hunan Province, China from January 2014 to December 2022.
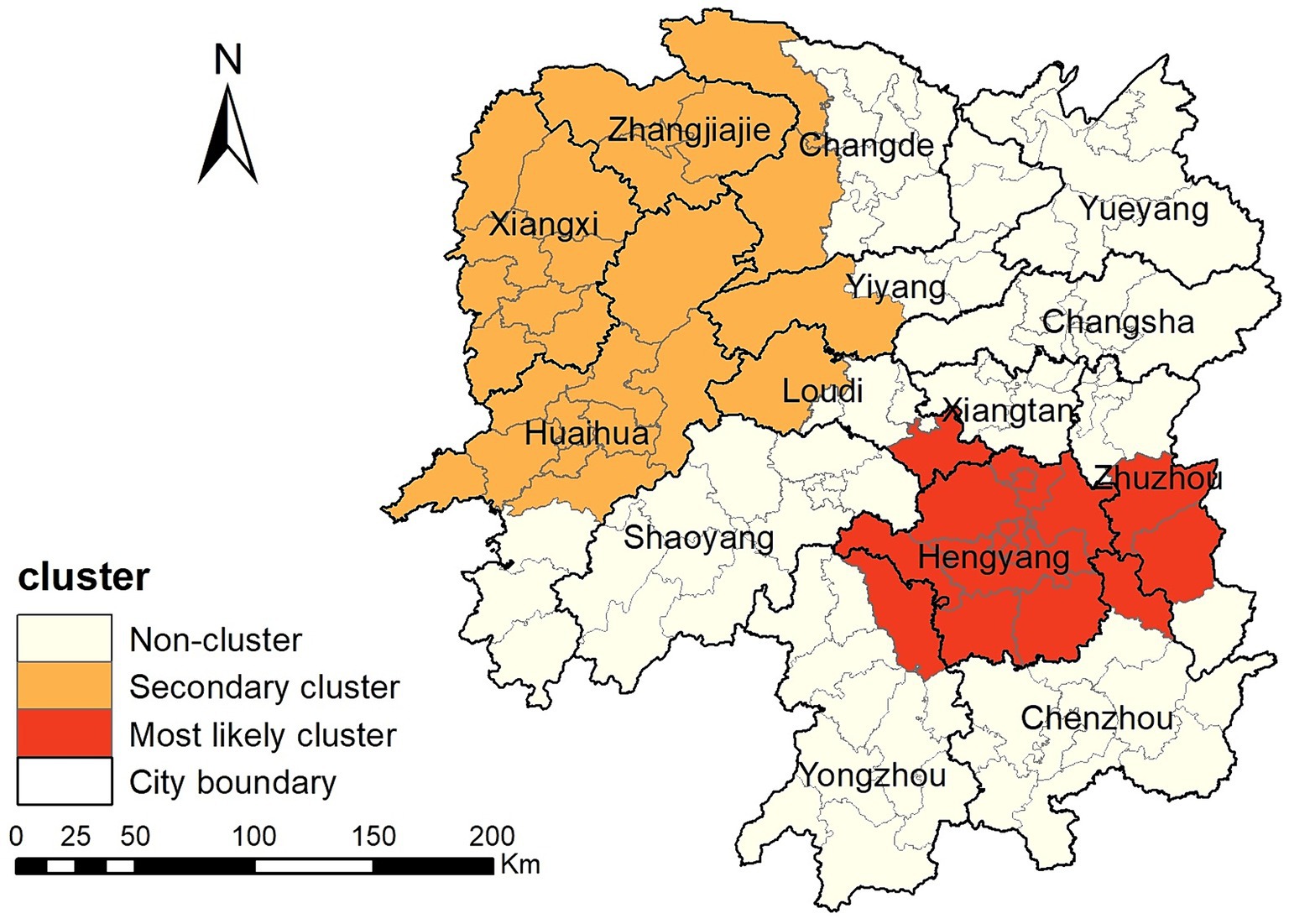
Figure 6 . Space-time clusters of cases of pulmonary tuberculosis at a county level in the Hunan Province from 2014 to 2022.
4 Discussion
This study found that the PTB notification rate decreased from 2014 to 2022 in the Hunan Province. PTB case notifications exhibited spatial clustering, with clustering occurring predominantly in the central-southern and northwestern regions of the province. The PTB notification rate in the Hunan Province decreased from 85.88/100,000 in 2014 to 66.40/100,000 in 2022, showing a slow downward trend with fluctuations. This declining trend mirrors observations made in various provinces and cities across China in a national study ( 26 ), including the Chongqing Municipality ( 13 ), Shandong Province ( 27 ), and Hubei Province ( 28 ). The decrease in the tuberculosis epidemic is mainly attributable to the efforts made by the national and local governments in tuberculosis control. The Hunan Province has implemented multiple measures to enhance its capacity for tuberculosis control across the province, establishing a novel tuberculosis control system. It actively conducts health education to raise awareness among the population about tuberculosis prevention and treatment. Molecular biology testing is widely promoted in prefectures and counties to achieve rapid diagnosis of PTB, leading to a gradual increase in the proportion of patients with microbiological confirmation and further improvement in the quality of tuberculosis diagnosis. Active screening is performed among key populations such as close contacts of patients with laboratory-confirmed PTB, older adults, and patients with diabetes patients to diagnose cases early, promptly initiate treatment, and reduce the spread of tuberculosis. In educational institutions, tuberculosis management has been strengthened by implementing PTB screening for students before entry into primary schools, middle schools, and high schools, as well as universities, controlling the entry of students with a high risk of developing tuberculosis. Additionally, preventive treatment for latent tuberculosis infection with a high risk of disease progression is promoted, reducing the occurrence of tuberculosis outbreaks in schools.
Our study found that the monthly PTB notifications showed significant seasonal variation, with peaks in January or March and troughs in December. The Hunan Province is located between 25° and 30° north latitude and has a subtropical monsoon climate. January is typically a winter month and is characterized by damp and rainy weather with little sunlight. Lack of exposure to ultraviolet light and poor ventilation are known risk factors for tuberculosis transmission ( 29 ). February coincides with the Chinese Lunar New Year holiday, when traditional customs discourage seeking medical care, leading to a higher PTB notification rate in March after the holiday period. This cyclical pattern is similar to research findings in the Chongqing Municipality ( 13 ), Hubei Province ( 28 ), Guangxi Province ( 30 ), and Hunan Province ( 15 ) but differs from the results observed in the Zhejiang Province ( 11 ), Taiwan ( 31 ) and Hong Kong ( 32 ). However, this pattern was disrupted from 2020 to 2022, with the peak in notifications delayed to June, April, and July in 2020, 2021, and 2022, respectively, although the trough still occurred in December each year. This may be attributed to the COVID-19 pandemic, which led to successive enforcement of control measures by the national and local governments based on the prevalence of COVID-19. People’s movements were affected, leading to delays in accessing healthcare services and diagnosis in individuals with PTB, thus disrupting the seasonal pattern of PTB notifications ( 33 , 34 ).
This study identified significant spatial clustering of PTB notifications in the Hunan Province, with hotspot areas primarily concentrated in the Xiangxi Autonomous Prefecture, Zhangjiajie City, and Hengyang City, similar to the findings of a previous study carried out in the Hunan Province in 2012 and 2013 ( 16 ). The hotspot areas in the Xiangxi Autonomous Prefecture gradually decreased over the years, disappearing by 2021 and 2022, whereas hotspot areas gradually formed in the Hengyang City from 2018 to 2022. Space-time scan analysis revealed that the most likely clustering area was around the Hengyang City, encompassing 17 adjacent counties and districts, with clustering occurring from March 1, 2018, to August 31, 2022. A secondary clustering area was identified around the Xiangxi Autonomous Prefecture and Zhangjiajie City, covering 25 adjacent counties and districts, with clustering occurring from January 1, 2014, to May 31, 2018. Local spatial autocorrelation analysis of hotspot areas was consistent with the clustering areas identified by space-time scan analysis, confirming the stability and reliability of the research results. The Xiangxi Autonomous Prefecture and Zhangjiajie City, located in the northwestern region of the province, are economically underdeveloped, with poor living conditions for residents and a lack of medical and health resources. Patients in these areas often come from impoverished families and may not receive timely diagnosis and treatment, causing the transmission of PTB and contributing to a higher incidence of the disease ( 35 , 36 ). In contrast, the Xiangxi Prefecture is an area in which ethnic minorities (Tujia and Miao) are concentrated. People belonging to ethnic minorities have an increased risk of delayed diagnosis of tuberculosis ( 37 ). In 2021, the PTB notification hotspot in the Zhangjiajie City decreased significantly, which may have been related to the COVID-19 epidemic in the Zhangjiajie City in July and August 2021 and the implementation of epidemic control measures. The COVID-19 pandemic resulted in reduced healthcare-seeking behavior among individuals with tuberculosis, affecting the diagnosis and treatment ( 34 , 38 ). This study also identified a cold-spot area that gradually expanded from the urban zones of Changsha, Zhuzhou, and Xiangtan in 2014 to encompass the entire city of Changsha, the urban zones of Zhuzhou and Xiangtan, and parts of Yueyang. This finding is generally similar to that of a previous study ( 15 ). These areas are the most economically developed region in the Hunan Province, with abundant medical resources and strong public awareness of disease prevention and treatment.
To our knowledge, this is the first space-time scan analysis of spatiotemporal clustering of PTB notifications at a county level in the Hunan Province. It identified regions with high PTB notification rates in the Hunan Province, providing valuable insights for formulating more effective control strategies.
This study has few limitations. First, the data were sourced from the National Disease Surveillance System, which may have resulted in an underestimation of the PTB epidemic in the Hunan Province due to some cases not being notified. Second, due to the adjustment of the national tuberculosis diagnostic criteria, cases of tuberculous pleurisy have been included in the pulmonary tuberculosis reporting system since May 1, 2018, which may introduce a slight bias in the research results. Third, this study used the space-time scan method to investigate PTB clustering. This method depends on circular spatial scan windows and space-time cylinders, which may not work well in irregular spaces, potentially leading to instability in determining clusters. Finally, this research examined only the spatiotemporal distribution patterns of tuberculosis without investigating individual factors and socioeconomic factors that may influence the incidence of tuberculosis. Some studies have found that personal factors such as smoking ( 39 ), alcohol consumption ( 40 , 41 ), HIV ( 42 ), and diabetes ( 43 ), as well as social factors such as poverty ( 36 ), population mobility ( 44 , 45 ), healthcare resources ( 46 ), and environmental pollution ( 47 , 48 ) may influence the distribution of tuberculosis. Future studies should aim to restrict the geographical unit of analysis to the township level and explore how these factors affect the incidence of PTB.
5 Conclusion
This study characterized the temporal trend and spatial distribution of tuberculosis in the Hunan Province. PTB diagnosis peaked in the spring. The PTB notification rate in the Hunan Province exhibited spatial clustering, concentrated mainly in the central-southern and northwestern regions of the province. This suggests that priority should be given to strengthening tuberculosis control efforts in these regions.
Data availability statement
The datasets presented in this article are not readily available because they are sourced from the Infectious Disease Information System and all data remain confidential. Requests to access the datasets should be directed to GH, [email protected] .
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Hunan Chest Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
GH: Data curation, Funding acquisition, Methodology, Software, Visualization, Writing – original draft. ZX: Data curation, Writing – review & editing. LB: Supervision, Writing – review & editing. JL: Conceptualization, Supervision, Writing – review & editing. SY: Software, Supervision, Writing – review & editing. HY: Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Natural Science Foundation of Hunan Province (grant number: 2021JJ40281).
Acknowledgments
The authors thank the staff at all Centers for Disease Control and Prevention, tuberculosis-designated hospitals, and community healthcare centers in Hunan Province for implementing tuberculosis reporting and notification in their daily work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization . Global Tuberculosis Report 2023 . Geneva: World Health Organization (2023).
Google Scholar
2. Xue, M, Zhong, J, Gao, M, Pan, R, Mo, Y, Hu, Y, et al. Analysis of spatial-temporal dynamic distribution and related factors of tuberculosis in China from 2008 to 2018. Sci Rep . (2023) 13:4974. doi: 10.1038/s41598-023-31430-0
Crossref Full Text | Google Scholar
3. Liu, MY, Li, QH, Zhang, YJ, Ma, Y, Liu, Y, Feng, W, et al. Spatial and temporal clustering analysis of tuberculosis in the mainland of China at the prefecture level, 2005–2015. Infect Dis Poverty . (2018) 7:106. doi: 10.1186/s40249-018-0490-8
4. Romanyukha, AA, Karkach, AS, Borisov, SE, Belilovsky, EM, Sannikova, TE, and Krivorotko, OI. Small-scale stable clusters of elevated tuberculosis incidence in Moscow, 2000–2015: Discovery and spatiotemporal analysis. Int J Infect Dis . (2020) 91:156–61. doi: 10.1016/j.ijid.2019.11.015
5. Lima, SVMA, Dos Santos, AD, Duque, AM, de Oliveira Goes, MA, da Silva Peixoto, MV, da Conceição, AD, et al. Spatial and temporal analysis of tuberculosis in an area of social inequality in Northeast Brazil. BMC Public Health . (2019) 19:873. doi: 10.1186/s12889-019-7224-0
6. Mesquita, CR, da Conceição, ML, de Oliveira, RAC, Conceição, EC, Garcez, JCD, Sousa, IFR, et al. Spatial analysis of tuberculosis patient flow in a neglected region of Northern Brazil. Trop Med Infect Dis . (2023):8. doi: 10.3390/tropicalmed8080397
7. Aceng, FL, Kabwama, SN, Ario, AR, Etwom, A, Turyahabwe, S, and Mugabe, FR. Spatial distribution and temporal trends of tuberculosis case notifications, Uganda: A ten-year retrospective analysis (2013–2022). BMC Infect Dis . (2024) 24:46. doi: 10.1186/s12879-023-08951-0
8. Huang, CC, Trevisi, L, Becerra, MC, Calderón, RI, Contreras, CC, Jimenez, J, et al. Spatial scale of tuberculosis transmission in Lima, Peru. Proc Natl Acad Sci USA . (2022) 119:e2207022119. doi: 10.1073/pnas.2207022119
9. Zhang, Y, Wang, XL, Feng, T, and Fang, CZ. Analysis of spatial-temporal distribution and influencing factors of pulmonary tuberculosis in China, during 2008–2015. Epidemiol Infect . (2018) 147:e25. doi: 10.1017/S0950268818002765
10. Li, L, Xi, Y, and Ren, F. Spatio-temporal distribution characteristics and trajectory similarity analysis of tuberculosis in Beijing, China. Int J Environ Res Public Health . (2016):13. doi: 10.3390/ijerph13030291
11. Ge, E, Zhang, X, Wang, X, and Wei, X. Spatial and temporal analysis of tuberculosis in Zhejiang Province, China, 2009–2012. Infect Dis Poverty . (2016) 5:11. doi: 10.1186/s40249-016-0104-2
12. Zhang, M, Chen, S, Luo, D, Chen, B, Zhang, Y, Wang, W, et al. Spatial-temporal analysis of pulmonary tuberculosis among students in the Zhejiang Province of China from 2007–2020. Front Public Health . (2023) 11:1114248. doi: 10.3389/fpubh.2023.1114248
13. Yu, Y, Wu, B, Wu, C, Wang, Q, Hu, D, and Chen, W. Spatial-temporal analysis of tuberculosis in Chongqing, China 2011–2018. BMC Infect Dis . (2020) 20:531. doi: 10.1186/s12879-020-05249-3
14. Rao, H, Shi, X, and Zhang, X. Using the Kulldorff’s scan statistical analysis to detect spatio-temporal clusters of tuberculosis in Qinghai Province, China, 2009–2016. BMC Infect Dis . (2017) 17:578. doi: 10.1186/s12879-017-2643-y
15. Alene, KA, Xu, Z, Bai, L, Yi, H, Tan, Y, Gray, DJ, et al. Spatiotemporal patterns of tuberculosis in Hunan Province, China. Int J Environ Res Public Health . (2021):18. doi: 10.3390/ijerph18136778
16. Zheng, J, Tang, Y, Zha, W, Liu, Y, Zhang, G, Hu, J, et al. Spatial distribution of tuberculosis in Hunan Province between 2012–2013: Analysis with geographic information system. Chin J Public Health . (2015) 31:1590–3. doi: 10.11847/zgggws2015-31-12-20
17. The People’s Government of Hunan Province, Introduction of Hunan . (2024). Available at: https://www.hunan.gov.cn/hnszf/jxxx/hngk/sqjs/sqjs.html (Accessed March 20, 2024).
18. National Health Commission of the People’s Republic of China . WS 288-2017 (2017). Beijing. Available at: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=0819ad84540b4d97a1644bbc6ec4306d (Accessed June 18, 2024).
19. National Health commission of the People’s Republic of China . Notice from the General Office of the National Health Commission on Adjusting the classification of infectious disease reports for pulmonary tuberculosis (2019). Beijing. Available at: http://www.nhc.gov.cn/jkj/s3589/201903/d779ae48db6446c28d1f5371ef09f5ab.shtml (Accessed March 1, 2024).
20. National Health commission of the People’s Republic of China . Notification of an adjustment to the classification of pulmonary tuberculosis reports (2017). Beijing. Available at: http://www.nhc.gov.cn/jkj/s3589/201707/e65698978c984013aa01b017f93dd75c.shtml (Accessed March 1, 2024).
21. Huo, XN, Li, H, Sun, DF, Zhou, LD, and Li, BG. Combining geostatistics with Moran’s I analysis for mapping soil heavy metals in Beijing, China. Int J Environ Res Public Health . (2012) 9:995–1017. doi: 10.3390/ijerph9030995
22. Anselin, L, Syabri, I, and Kho, Y. GeoDa: An introduction to spatial data analysis. Geogr Anal . (2006) 38:5–22. doi: 10.1111/j.0016-7363.2005.00671.x
23. Getis, A, and Ord, J. The analysis of spatial association by use of distance statistics. Geogr Analys . (1995) 27:93–115.
24. Kulldorff, M . A spatial scan statistic. Commun. Stat . (1997) 26:1481–96. doi: 10.1080/03610929708831995
25. Deng, T, Huang, Y, Yu, S, Gu, J, Huang, C, Xiao, G, et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS One . (2013) 8:e56943. doi: 10.1371/journal.pone.0056943
26. Dong, Z, Wang, QQ, Yu, SC, Huang, F, Liu, JJ, Yao, HY, et al. Age-period-cohort analysis of pulmonary tuberculosis reported incidence, China, 2006–2020. Infect Dis Poverty . (2022) 11:85. doi: 10.1186/s40249-022-01009-4
27. Duan, Y, Cheng, J, Liu, Y, Fang, Q, Sun, M, Cheng, C, et al. Epidemiological characteristics and spatial-temporal analysis of tuberculosis at the county-level in Shandong Province, China, 2016–2020. Trop Med Infect Dis . (2022) 7:346. doi: 10.3390/tropicalmed7110346
PubMed Abstract | Crossref Full Text | Google Scholar
28. Zhang, Y, Ye, J, Hou, S, Lu, X, Yang, C, Pi, Q, et al. Spatial-temporal analysis of pulmonary tuberculosis in Hubei Province, China, 2011–2021. PLoS One . (2023) 18:e0281479. doi: 10.1371/journal.pone.0281479
29. Thorpe, LE, Frieden, TR, Laserson, KF, Wells, C, and Khatri, GR. Seasonality of tuberculosis in India: Is it real and what does it tell us? Lancet . (2004) 364:1613–4. doi: 10.1016/S0140-6736(04)17316-9
30. Cui, Z, Lin, D, Chongsuvivatwong, V, Zhao, J, Lin, M, Ou, J, et al. Spatiotemporal patterns and ecological factors of tuberculosis notification: A spatial panel data analysis in Guangxi, China. PLoS ONE . (2019) 14:e0212051. doi: 10.1371/journal.pone.0212051
31. Liao, CM, Hsieh, NH, Huang, TL, Cheng, YH, Lin, YJ, Chio, CP, et al. Assessing trends and predictors of tuberculosis in Taiwan. BMC Public Health . (2012) 12:29. doi: 10.1186/1471-2458-12-29
32. Leung, CC, Yew, WW, Chan, TYK, Tam, CM, Chan, CY, Chan, CK, et al. Seasonal pattern of tuberculosis in Hong Kong. Int J Epidemiol . (2005) 34:924–30. doi: 10.1093/ije/dyi080
33. Zhang, Y, Zhang, L, Gao, W, Li, M, Luo, Q, Xiang, Y, et al. The impact of COVID-19 pandemic on reported tuberculosis incidence and mortality in China: An interrupted time series analysis. J Glob Health . (2023) 13:06043. doi: 10.7189/jogh.13.06043
34. Xu, J, Wang, Y, Liu, F, and Yang, H. Changes of tuberculosis infection in mainland China before and after the COVID-19 pandemic. J Infect . (2023) 86:154–225. doi: 10.1016/j.jinf.2022.12.008
35. Mou, J, Griffiths, SM, Fong, H, and Dawes, MG. Health of China’s rural-urban migrants and their families: A review of literature from 2000 to 2012. Br Med Bull . (2013) 106:19–43. doi: 10.1093/bmb/ldt016
36. Huang, L, Li, XX, Abe, EM, Xu, L, Ruan, Y, Cao, CL, et al. Spatial-temporal analysis of pulmonary tuberculosis in the northeast of the Yunnan Province, People’s Republic of China. Infect Dis Poverty . (2017) 6:53. doi: 10.1186/s40249-017-0268-4
37. Gilmour, B, Xu, Z, Bai, L, Alene, KA, and Clements, ACA. The impact of ethnic minority status on tuberculosis diagnosis and treatment delays in Hunan Province China. BMC Infect Dis . (2022) 22:90. doi: 10.1186/s12879-022-07072-4
38. Zhang, G, Yu, Y, Zhang, W, Shang, J, Chen, S, Pang, X, et al. Influence of COVID-19 for delaying the diagnosis and treatment of pulmonary tuberculosis-Tianjin, China. Front Public Health . (2022) 10:937844. doi: 10.3389/fpubh.2022.937844
39. Feng, Y, Xu, Y, Yang, Y, Yi, G, Su, H, Chen, H, et al. Effects of smoking on the severity and transmission of pulmonary tuberculosis: A hospital-based case control study. Front Public Health . (2023) 11:1017967. doi: 10.3389/fpubh.2023.1017967
40. Weiangkham, D, Umnuaypornlert, A, Saokaew, S, Prommongkol, S, and Ponmark, J. Effect of alcohol consumption on relapse outcomes among tuberculosis patients: A systematic review and meta-analysis. Front Public Health . (2022) 10:962809. doi: 10.3389/fpubh.2022.962809
41. Wigger, GW, Bouton, TC, Jacobson, KR, Auld, SC, Yeligar, SM, and Staitieh, BS. The impact of alcohol use disorder on tuberculosis: A review of the epidemiology and potential immunologic mechanisms. Front Immunol . (2022) 13:864817. doi: 10.3389/fimmu.2022.864817
42. Zou, S, Tan, Y, Xiang, Y, Liu, Y, Zhu, Q, Wu, S, et al. The role of CD4+CD8+ T cells in HIV infection with tuberculosis. Front Public Health . (2022) 10:895179. doi: 10.3389/fpubh.2022.895179
43. Ssekamatte, P, Sande, OJ, van Crevel, R, and Biraro, IA. Immunologic, metabolic and genetic impact of diabetes on tuberculosis susceptibility. Front Immunol . (2023) 14:1122255. doi: 10.3389/fimmu.2023.1122255
44. Zhang, H, Sun, R, Wu, Z, Liu, Y, Chen, M, Huang, J, et al. Spatial pattern of isoniazid-resistant tuberculosis and its associated factors among a population with migrants in China: A retrospective population-based study. Front Public Health . (2024) 12:1372146. doi: 10.3389/fpubh.2024.1372146
45. Liu, K, Chen, S, Zhang, Y, Li, T, Xie, B, Wang, W, et al. Tuberculosis burden caused by migrant population in Eastern China: Evidence from notification records in Zhejiang Province during 2013–2017. BMC Infect Dis . (2022) 22:109. doi: 10.1186/s12879-022-07071-5
46. Pradipta, IS, Idrus, LR, Probandari, A, Puspitasari, IM, Santoso, P, Alffenaar, JC, et al. Barriers to optimal tuberculosis treatment services at community health centers: A qualitative study from a high prevalent tuberculosis country. Front Pharmacol . (2022) 13:857783. doi: 10.3389/fphar.2022.857783
47. Nardell, EA . Indoor environmental control of tuberculosis and other airborne infections. Indoor Air . (2016) 26:79–87. doi: 10.1111/ina.12232
48. Li, JX, Luan, Q, Li, B, Dharmage, SC, Heinrich, J, Bloom, MS, et al. Outdoor environmental exposome and the burden of tuberculosis: Findings from nearly two million adults in northwestern China. J Hazard Mater . (2023) 459:132222. doi: 10.1016/j.jhazmat.2023.132222
Keywords: tuberculosis, spatiotemporal analysis, spatial autocorrelation analysis, clustering, China
Citation: Huang G, Xu Z, Bai L, Liu J, Yu S and Yao H (2024) Spatiotemporal analysis of tuberculosis in the Hunan Province, China, 2014–2022. Front. Public Health . 12:1426503. doi: 10.3389/fpubh.2024.1426503
Received: 01 May 2024; Accepted: 30 July 2024; Published: 08 August 2024.
Reviewed by:
Copyright © 2024 Huang, Xu, Bai, Liu, Yu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Yao, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

COMMENTS
Read chapter 34 of Infectious Diseases: A Case Study Approach online now, exclusively on AccessPharmacy. AccessPharmacy is a subscription-based resource from McGraw Hill that features trusted pharmacy content from the best minds in the field. ... Tuberculosis. In: Cho JC. Cho J.C.(Ed.), Ed. Jonathan C. Cho. eds. Infectious Diseases: A Case ...
Introduction. Tuberculosis (TB) is caused by strains of Mycobacterium tuberculosis (M. Tuberculosis). TB is a primarily pulmonary infection spread by airborne droplet transmission. The development and spread of drug-resistant strains of M. tuberculosis greatly jeopardize TB control efforts. In the US, 91 cases of MDR-TB were reported in 2014.
Clinical and radiological manifestations of tuberculosis (TB) are heterogeneous, and differential diagnosis can include both benign and malignant diseases (e.g., sarcoidosis, metastatic diseases, and lymphoma). Diagnostic dilemmas can delay appropriate therapy, favoring Mycobacterium tuberculosis transmission.We report on a case of TB in an immunocompetent, Somalian 22-year-old boy admitted in ...
Case Study. A 44-year-old man presented to the TB Clinic with symptoms of progressive shortness of breath and cough with greenish sputum production. His sputum test results showed that he had atypical TB ( Mycobacterium Avium Complex MAC infection ). He was HIV negative at this time. Past history revealed that he was in good health till 1991 ...
We systematically reviewed the literature for studies that reported the effects of active case-finding interventions on tuberculosis epidemiological indicators. Our literature search was an update of a 2013 systematic review by Kranzer and colleagues, 3 which covered the period between Jan 1, 1980, and Oct 13, 2010, with additional searches by ...
Background Mycobacterium tuberculosis (Mtb) has been found to persist within cavities in patients who have completed their anti-tuberculosis therapy. The clinical implications of Mtb persistence after therapy include recurrence of disease and destructive changes within the lungs. Data on residual changes in patients who completed anti-tuberculosis therapy are scarce. This case highlights the ...
Patients with highly drug-resistant forms of tuberculosis have limited treatment options and historically have had poor outcomes. In an open-label, single-group study in which follow-up is ongoing ...
Although tuberculosis (TB) is a curable disease, treatment is complex and prolonged, requiring considerable commitment from patients. This study aimed to understand the common perspectives of TB patients across Brazil, Russia, India, China, and South Africa throughout their disease journey, including the emotional, psychological, and practical challenges that patients and their families face.
Studies of active case-finding in the general population, in populations perceived to be at high risk for tuberculosis, and in closed settings were included, whereas studies of tuberculosis screening at health-care facilities, among household contacts, or among children only, and studies that screened fewer than 1000 people were excluded.
The Western Pacific Region has one of the fastest-growing populations of older adults (≥ 65 years) globally, among whom tuberculosis (TB) poses a particular concern. This study reports country case studies from China, Japan, the Republic of Korea, and Singapore reflecting on their experiences in managing TB among older adults. Across all four countries, TB case notification and incidence ...
Case Study 3 TB Infection with Adverse Drug Reaction.....31 Participants will learn about the persons who are at high risk for exposure to tuberculosis, the current methods for diagnosing latent TB infection, treatment of patients with latent tuberculosis infection, and potential adverse reactions and responses to medications used ...
Personal Stories from TB Survivors - My Journey fighting TB. 3 June 2020. Tuberculosis (TB) is the world's top infectious killer today. It is airborne and can affect any one of us. Over 5 000 women, men and children still die each day from TB. The social and economic impacts are devastating, including poverty, stigma and discrimination.
report findings of a systematic review summarising the evidence for population-level effectiveness of active case-finding for tuberculosis. The authors appraised 36 studies published between Jan 1, 1980, and April 13, 2020, of adult populations from 16 countries that were exposed to different active case-finding interventions.
case study on pulmonary tubercul osis Vedha Pal Jeyamani.S 1 , Angel.P 1 , Bhavya.E 1 , Balaji.P 1 , Muruga n.M 2 1 Department of Pharmacology , Ja ya College of Pharmacy, Chennai, T amilnadu, India
Three outcomes were evaluated: case notification rates (which were expected to increase3), prevalence of active tuberculosis, and tuber culosis infection among young children as an indicator of transmission (both expected to decrease in the long term).3 Case notification rates were assessed in 28 studies, of which six were randomised trials.
Lesson 3: Testing for TB Infection. HOME. GLOSSARY. EXIT. NEXT. BACK. Centers for Disease Control and Prevention 1600 Clifton Rd. Atlanta, GA 30329, USA ... Page 19 of 23. CASE STUDY. Now that you have learned how people are tested for TB, apply your knowledge with the following case study. ...
Digital, case-based, real-time surveillance for TB: status of progress. Tuberculosis (TB) surveillance is the continuous and systematic collection, analysis and reporting of data related to TB infection and TB disease in the population. To support countries to implement national surveillance systems for TB in a consistent and comparable way ...
improve adherence. A case study highlights patient-centered TB case management. Chapter 3: Tuberculosis Nurse Case Management in Special Situations and Circumstances This chapter provides information and case studies on TB in selected situations and circumstances, ranging from TB in people experiencing homelessness to those with TB and diabetes.
CASE STUDY. David's health care provider refers him to the local health department for further evaluation. At the health department, David answers questions about his medical history and current medications. He also receives a chest x-ray, a physical examination, and coughs some sputum into a container.
This study reports country case studies from China, Japan, the Republic of Korea, and Singapore, reflecting on country-specific experiences in TB diagnosis and management among older adults. The case studies are a part of a broader endeavour, including a narrative review and analysis of epidemiological trends, to understand and document TB ...
TB Case study. TB CASE STUDY #1. TB- RESPIRATORY ISOLATION. 20XX PRESENTATION TITLE 2. A 31-year-old caucasian male presented to the Emergency Department (ED) after experiencing gross hemoptysis. He had a 2 month history of productive cough, a 25 pound weight loss, night sweats, and fatigue. A CXR revealed bilateral cavitary infiltrates.
On average, across study sites, 69% of TB cases and 48% of close contacts were born in a country with a high TB incidence, defined as >30 per 100,000 TB incidence based on the World Health ...
As per the India TB Report 2022, 22.1 crore individuals in India were screened for presumptive TB as part of active case-finding. Of these, only 48,329 (2.5%) were diagnosed, resulting in a low ...
Lesson 6: Treatment of TB Disease. HOME. GLOSSARY. EXIT. NEXT. BACK. Centers for Disease Control and Prevention 1600 Clifton Rd. Atlanta, GA 30329, USA ... Lesson 6 Case Study. Page 20 of 29. CASE STUDY. Now that you have learned about the treatment of TB disease, check your knowledge with the following case study. ...
Diagnosing disseminated TB is difficult as it mimics conditions, such as infarction, cancer, torsion, etc, so attention to small details is necessary. Background: Disseminated tuberculosis (TB) is the presence of two or more noncontiguous sites resulting from hematogenous dissemination of Mycobacterium tuberculosis. We report a case of disseminated TB with testicular involvement. Case: A 21 ...
A complete genetic study of these strains led to their integration into the M. tuberculosis complex. This strain, identified as M. tuberculosis subsp. canetti or, more simply, M. canetti, was first isolated in 1969 by Georges Canetti from a French farmer. ... In 1997, van Soolingen reported a case of lymph node TB in a 2-year-old Somali child ...
Delays in the diagnosis and treatment of pulmonary tuberculosis (PTB) can increase the risk of transmission, thereby posing a significant risk to public health. Early diagnosis is considered to play a crucial role in eliminating TB. Rapid testing, active case finding, and health education are effective strategies for reducing tuberculosis diagnosis delays (TDDs). This study aimed to ...
In our study, another factor influencing case fatality was the proportion of females, which suggests that as the proportion of females increases, so does the case fatality rate. The worldwide incidence of TB has consistently been higher in men compared with women, although the male-to-female ratio varies per region [ 25 ].
From 2014-2016, an unprecedented tuberculosis outbreak occurred in Marion County in Alabama, resulting in an incident rate worse than experienced in many low and middle-income countries. ... The case study concludes that structural racism in healthcare settings must be addressed to prevent similar outbreaks from occurring in the future. ...
Therefore, this study investigated the spatiotemporal distribution of PTB in the Hunan Province to enable targeted control policies for tuberculosis. Methods: We obtained data about cases of PTB in the Hunan Province notified from January 2014 to December 2022 from the China Information System for Disease Control and Prevention.