Talk to our experts
1800-120-456-456
- De Broglie Equation


Introduction
The wave nature of light was the only aspect that was considered until Neil Bohr’s model. Later, however, Max Planck in his explanation of quantum theory hypothesized that light is made of very minute pockets of energy which are in turn made of photons or quanta. It was then considered that light has a particle nature and every packet of light always emits a certain fixed amount of energy.
By this, the energy of photons can be expressed as:
E = hf = h * c/λ
Here, h is Plank’s constant
F refers to the frequency of the waves
Λ implies the wavelength of the pockets
Therefore, this basically insinuates that light has both the properties of particle duality as well as wave.
Louis de Broglie was a student of Bohr, who then formulated his own hypothesis of wave-particle duality, drawn from this understanding of light. Later on, when this hypothesis was proven true, it became a very important concept in particle physics.
⇒ Don't Miss Out: Get Your Free JEE Main Rank Predictor 2024 Instantly! 🚀
What is the De Broglie Equation?
Quantum mechanics assumes matter to be both like a wave as well as a particle at the sub-atomic level. The De Broglie equation states that every particle that moves can sometimes act as a wave, and sometimes as a particle. The wave which is associated with the particles that are moving are known as the matter-wave, and also as the De Broglie wave. The wavelength is known as the de Broglie wavelength.
For an electron, de Broglie wavelength equation is:
λ = \[\frac{h}{mv}\]
Here, λ points to the wave of the electron in question
M is the mass of the electron
V is the velocity of the electron
Mv is the momentum that is formed as a result
It was found out that this equation works and applies to every form of matter in the universe, i.e, Everything in this universe, from living beings to inanimate objects, all have wave particle duality.
Significance of De Broglie Equation
De Broglie says that all the objects that are in motion have a particle nature. However, if we look at a moving ball or a moving car, they don’t seem to have particle nature. To make this clear, De Broglie derived the wavelengths of electrons and a cricket ball. Now, let’s understand how he did this.
De Broglie Wavelength
1. De Broglie Wavelength for a Cricket Ball
Let’s say,Mass of the ball = 150 g (150 x 10⁻³ kg),
Velocity = 35 m/s,
and h = 6.626 x 10⁻³⁴ Js
Now, putting these values in the equation
λ = (6.626 * 10 to power of -34)/ (150 * 10 to power of -3 *35)
This yields
λBALL = 1.2621 x 10 to the power of -34 m,
Which is 1.2621 x 10 to the power of -24 Å.
We know that Å is a very small unit, and therefore the value is in the power of 10−24−24^{-24}, which is a very small value. From here, we see that the moving cricket ball is a particle.
Now, the question arises if this ball has a wave nature or not. Your answer will be a big no because the value of λBALL is immeasurable. This proves that de Broglie’s theory of wave-particle duality is valid for the moving objects ‘up to’ the size (not equal to the size) of the electrons.
De Broglie Wavelength for an Electron
We know that me = 9.1 x 10 to power of -31 kg
and ve = 218 x 10 to power of -6 m/s
Now, putting these values in the equation λ = h/mv, which yields λ = 3.2 Å.
This value is measurable. Therefore, we can say that electrons have wave-particle duality. Thus all the big objects have a wave nature and microscopic objects like electrons have wave-particle nature.
E = hν = \[\frac{hc}{\lambda }\]
The Conclusion of De Broglie Hypothesis
From de Broglie equation for a material particle, i.e.,
λ = \[\frac{h}{p}\]or \[\frac{h}{mv}\], we conclude the following:
i. If v = 0, then λ = ∞, and
If v = ∞, then λ = 0
It means that waves are associated with the moving material particles only. This implies these waves are independent of their charge.

FAQs on De Broglie Equation
1.The De Broglie hypothesis was confirmed through which means?
De Broglie had not proved the validity of his hypothesis on his own, it was merely a hypothetical assumption before it was tested out and consequently, it was found that all substances in the universe have wave-particle duality. A number of experiments were conducted with Fresnel diffraction as well as a specular reflection of neutral atoms. These experiments proved the validity of De Broglie’s statements and made his hypothesis come true. These experiments were conducted by some of his students.
2.What exactly does the De Broglie equation apply to?
In very broad terms, this applies to pretty much everything in the tangible universe. This means that people, non-living things, trees and animals, all of these come under the purview of the hypothesis. Any particle of any substance that has matter and has linear momentum also is a wave. The wavelength will be inversely related to the magnitude of the linear momentum of the particle. Therefore, everything in the universe that has matter, is applicable to fit under the De Broglie equation.
3.Is it possible that a single photon also has a wavelength?
When De Broglie had proposed his hypothesis, he derived from the work of Planck that light is made up of small pockets that have a certain energy, known as photons. For his own hypothesis, he said that all things in the universe that have to matter have wave-particle duality, and therefore, wavelength. This extends to light as well, since it was proved that light is made up of matter (photons). Hence, it is true that even a single photon has a wavelength.
4.Are there any practical applications of the De Broglie equation?
It would be wrong to say that people use this equation in their everyday lives, because they do not, not in the literal sense at least. However, practical applications do not only refer to whether they can tangibly be used by everyone. The truth of the De Broglie equation lies in the fact that we, as human beings, also are made of matter and thus we also have wave-particle duality. All the things we work with have wave-particle duality.
5.Does the De Broglie equation apply to an electron?
Yes, this equation is applicable for every single moving body in the universe, down to the smallest subatomic levels. Just how light particles like photons have their own wavelengths, it is also true for an electron. The equation treats electrons as both waves as well as particles, only then will it have wave-particle duality. For every electron of every atom of every element, this stands true and using the equation mentioned, the wavelength of an electron can also be calculated.
6.Derive the relation between De Broglie wavelength and temperature.
We know that the average KE of a particle is:
K = 3/2 k b T
Where k b is Boltzmann’s constant, and
T = temperature in Kelvin
The kinetic energy of a particle is ½ mv²
The momentum of a particle, p = mv = √2mK
= √2m(3/2)KbT = √2mKbT
de Broglie wavelength, λ = h/p = h√2mkbT
7.If an electron behaves like a wave, what should determine its wavelength and frequency?
Momentum and energy determine the wavelength and frequency of an electron.
8. Find λ associated with an H 2 of mass 3 a.m.u moving with a velocity of 4 km/s.
Here, v = 4 x 10³ m/s
Mass of hydrogen = 3 a.m.u = 3 x 1.67 x 10⁻²⁷kg = 5 x 10⁻²⁷kg
On putting these values in the equation λ = h/mv we get
λ = (6.626 x 10⁻³⁴)/(4 x 10³ x 5 x 10⁻²⁷) = 3 x 10⁻¹¹ m.
9. If the KE of an electron increases by 21%, find the percentage change in its De Broglie wavelength.
We know that λ = h/√2mK
So, λ i = h/√(2m x 100) , and λ f = h/√(2m x 121)
% change in λ is:
Change in wavelength/Original x 100 = (λ fi - λ f )/λ i = ((h/√2m)(1/10 - 1/21))/(h/√2m)(1/10)
On solving, we get
% change in λ = 5.238 %
- Wave Nature of Matter
What does wave nature of matter mean? Can a small particle be at multiple places at the same time? Do I have a wave nature? Why can’t I see it? Let’s try to answer these questions.
Suggested Videos
In the earlier articles, we saw how light behaves both as a wave and particle. A particle is confined at a place. On the other hand, a wave is spread in space. We say that the nature of light depends on the nature of our observation. If you are observing phenomenon like the interference, diffraction or reflection, you will find that light is a wave. However, if you are looking at phenomena like the photoelectric effect, you will find that light has a particle character.
You might ask, which is it? Is light a wave or a particle? The answer is that it has a dual nature. You may also wonder whether it is a specific property of light! Does only light have a dual nature? What if other quantities had dual nature? How could we measure and prove that? Maybe these were the questions that led Louis Victor de Broglie to come up with one of the most revolutionary equations in Physics, the de Broglie equation.
Browse more Topics under Dual Nature Of Radiation And Matter
- Electron Emission
- Experimental Study of Photoelectric Effect
- Davisson and Germer Experiment
- Einstein’s Photoelectric Equation: Energy Quantum of Radiation
Next up – A Few Lines of Math That Go A Long Way!
Let us recall the mass-energy equivalence of Einstein, E =mc 2 …(1)
Also from Einstein-Plank relation, we have: E = hν …(2)
Furthermore, we see that equation (1) is applicable to particles with some “mass”. In other words equation (1) can be applied to particles and equation (2) is an equation for a wave of frequency ν. So the two were not equated until de Broglie had a breakthrough! We know that light can be a wave as well as a particle. In that case, we can say that equation (1) and (2) represent the same quantity. Consequently, we must have: hν = mc 2 . Since we know that ν = c/λ, we have:
h(c/λ) = mc 2
λ = h/mc; where ‘c’ is the velocity of light. If we have a wave of velocity, say ‘v’, we can write: λ = h/mv
or λ = h/p …(3)
where ‘p’ is the momentum of the wave-particle! See what we did here? We have mass – a particle property, in the same equation as wavelength – a wave property. Thus if matter exhibits wave properties, it must be given by equation (3). Equation (3) is the de Broglie equation and represents the wave-particle duality. Hence we say that everything in the Cosmos exhibits a dual nature. This is the wave nature of radiation and matter.
Wavelength of Macroscopic Objects
“So you are telling me that I am not a particle but a wave? Where is it then?” First of all, you are both. Let us find out your wavelength. Suppose you have a mass of 55 kg. If you are at rest i.e. if the velocity = 0, then we see from equation (3), that λ is not defined. So not much help there! Let us say that you are moving at a velocity of 5 m/s. Using equation (3), we can see that
λ = h/(55)×5
λ = 6.63×10-34/275 ≈ 2.4×10-36 m
As you can see, you can’t “see” this small wavelength. Thus the wavelength of macroscopic objects is too small to have any observable effects on any property at normal velocities.
Learn more about Wave Optics .
So de Broglie Guessed An Equation And Everyone Just Agreed?
Fortunately, there was a way to verify this equation. Let us see the equation again, λ = h/p
We know that K.E. = 1/2(mv 2 )
or K.E. = \( \frac{(mv)^2}{(2m)} \) = \( \frac{(p)^2}{(2m)} \)
Here, p is the momentum. Thus we have: p = \( \sqrt[]{2mE} \) …. (4)
Using (4) in (3), we have: λ = h/\( \sqrt[]{2mE} \) …(5)
Also for a charged particle, E = eV and we have: λ = h/\( \sqrt[]{2meV} \)
So for an electron e = 1.6×10 -19 C and m = 9.10938356 × 10 -31 kilograms, we have:
Hence we can verify the de Broglie equation if we observe the motion of an electron. This was done in the Davisson and Germer Experiment.
Solved Examples For You
The de-Broglie wavelength of an electron (mass 1 × 10 − 30 k g , charge = 1.6 × 10 − 19 C ) with a kinetic energy of 200 e V is: (Planck’s constant 6.6 × 10 − 34 J ):
A) 9.60 × 10 − 11 m B) 8.25 × 10 − 11 m
C) 6.25 × 10 − 11 m C) 5.00 × 10 − 11 m
Solution: B) 8.25 × 10 − 11 m
We can directly use equation (5) i.e. λ = h/\( \sqrt[]{2mE} \). Substitution of the respective values gives the required result.
Customize your course in 30 seconds
Which class are you in.

Dual Nature of Radiation and Matter
- Radiation Detector
- Cherenkov Radiation
- Einstein’s Photoelectric Equation: Energy Quantum of Radiation
One response to “Davisson and Germer Experiment”
Eassy understand
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Revision notes for IB Physics
Topic 12: quantum and nuclear physics (hl).
See the guide for this topic.
12.1 – The interaction of matter with radiation
- Einstein proposed that light consists of particles called photons.
- Quantum refers to the smallest discrete amount of something. A photon is a quantum of electromagnetic radiation (light).
- Photons exhibit wave properties under refraction or interference.
- Photons exhibit wave properties under its emission or absorption.
- A photon’s energy (E) is proportional to its frequency (f) and is given by
where h is Planck’s constant, c is the speed of light, and λ is its wavelength (electromagnetic wave).
The photoelectric effect
- Photoelectric effect refers to the emission of electrons from a metal surface as a result of the absorption of electromagnetic wave energy.

| Intensity | Affects the number of electrons ejected. |
| Frequency | There exists a minimum frequency (depending on the material) below which no electrons are ejected. Affects the maximum KE of ejected electrons |
- An example of the photoelectric effect on a sample metal surface.

Incident electromagnetic waves with lower frequency have a smaller chance of inducing the photoelectric effect.
- Why does the intensity of light affect the number of ejected electrons?
The number of photons per unit time in the incident light is proportional to the light intensity.
An increase in the intensity of the incident light allows a higher number of photon-electron interactions. Therefore, more electrons are ejected.
- Why is there a minimum frequency below which no electrons are ejected?
There exists a minimum energy below which electrons would not be ejected from the metal. This minimum energy level depends on the metal in use and is called the work function (φ).
Since E=hf, φ=hf0 where f0 is called the threshold frequency.
- How does the frequency of the incident light affect the maximum kinetic energy of the ejected electrons?
The work function corresponds to the potential energy which binds the electron to the nucleus.
Since total energy = potential energy + kinetic energy,
which may be represented on graph by the following

Matter waves
- The De Broglie hypothesis suggests that all matter exhibits wave-like properties. In particular, the momentum of a particle is related to its wavelength where the De Broglie wavelength may be deduced by the following formula
where p is momentum, h is Planck’s constant, λ is wavelength, m is mass, and v is velocity.
- The term “wave-particle duality” refers to matter acting as both waves and particles.
Pair production and pair annihilation
All matters have their antimatter counterparts which resemble their corresponding matter in every way except for the sign of their charge and the direction of their spin.
Pair production
When a high energy photon collides with a nucleus, it makes a pair of electron and positron (electron antimatter) and gives kinetic energy to each particle.

Pair annihilation
When matter collides with its corresponding antimatter, they annihilate one another with the conservation of energy, momentum, and charge.

The positron (+e) collides with the electron (-e), annihilating each other into two photons with exactly opposite directions and the same amount of momentum.
Quantization of angular momentum in the Bohr model for hydrogen
- Bohr developed a model for hydrogen that was able to explain the emission and absorption spectra of hydrogen.
- His model assumed discrete orbital paths in which electrons orbit the nucleus through, the same way planets orbit stars.
- The orbits were quantized in terms of their allowable angular momentum (rotational momentum).
- Therefore, the orbital radii and energies are also quantized.
- The energy of the orbit is the energy required to ionize (remove) an electron and can be given through the following equation in relation to the order of orbit (n)

- When the electrons are excited, they jump to higher energy orbits and eventually drop back down to a more stable orbit by releasing excess energy by the form of light. The energy of the light released is therefore equal to the difference in energy of the two orbits.
The wave function
By quantum physics, all particles do not have a defined position until they are observed. Instead, all particles are described as “a wave function”.
TL;DR : The wave function gives the probability of finding a particle at a given point which is given by the square of the amplitude of the wave function at that location.
The uncertainty principle for energy and time and position and momentum
The Heisenberg uncertainty principle states that
- If the energy state only lasts for a brief period of time, its energy is uncertain.
- Position and momentum cannot be measured simultaneously with precision. The more precisely the position is determined, the less precisely the momentum is known, and vice versa.

Tunnelling, potential barrier and factors affecting tunnelling probability
- Imagine throwing a ball at a wall and having it disappear the instant before making contact and appearing on the other side. The wall remains intact and the ball did not break through it. Believe it or not, there is a finite (if extremely small) probability that this even would occur. This phenomenon is called quantum tunnelling.

- The position of a particle is described as a wave function (see previous section).
- From the graph above, the observable particle is most likely to be at the position where its wave function has the largest amplitude. However, although the amplitude of the wave function will decay exponentially, since the wave function does not reach an amplitude of zero, the wave function can exit the barrier. Once the wave function exits the barrier, its amplitude no longer decays. This means that a particle has a certain probability of bouncing off a barrier and a certain probability of passing through the other side.
| Increase barrier length | Decrease |
| Increase particle mass | Decrease |
- This explains how tunnelling is frequent in nanoscale but negligible at the macroscopic level.
12.2 – Nuclear physics
Rutherford scattering and nuclear radius.
Rutherford’s undergraduate students, Geiger and Marsden, bombarded a sheet of gold foil by alpha particles.
The alpha particles passed through the gold foil in most cases, a small percentage of alpha particles were deflected by small angles of deflection, and an even smaller percentage of alpha particles were deflected by large angles of deflection.
Rutherford thus deduced that the atom consists of a small compact positive nucleus (where alpha particles deflect by large angles) with a majority of volume existing as empty space (where alpha particles pass right through).

Nuclear energy levels
- In the same way electrons can move between discrete energy levels, the nucleus of an atom can too.
- Atoms that decay through gamma decay emit distinct frequencies of gamma rays which correspond to distinct energy levels.
The neutrino
- A neutrino is a type of lepton. Since they have no electrical charge or strong charge, most neutrinos do not react with other particles and pass right through earth with no interaction.
- Neutrinos are produced in many particle decays, such as in beta decay. When a neutron at rest (zero momentum) decays by releasing a proton and an electron, because of the law of conservation of momentum, the resultant products of decay must have a total momentum of zero, which the observed proton and electron clearly does not portray. Therefore, we suggest the presence of another particle to balance the momentum – by the release of an antineutrino (neutrino antimatter). This was confirmed by experimentation.

- Neutrinos were produced in great abundance in the early universe and rarely interact with matter. This may suggest that neutrinos contribute to the total mass of the universe and affects its expansion.
The law of radioactive decay and the decay constant
Apart from half-lives (see topic 7), the activity of radioactive decay can also be shown exponentially by the law of radioactive decay.

- The decay constant (λ) represents the probability of decay of a nucleus per unit time and is dependent on the type of element.

Share this:
Explain de-Broglie wavelength. - Physics
Advertisements.
Explain de-Broglie wavelength.
Solution Show Solution
The wavelength that is associated with an object in relation to its momentum and mass is known as the de-Broglie wavelength.
de-Broglie equated the energy equation of Plank (wave nature) and Einstein (particle nature) such that,
E = hv (Plank energy relation)
E = mc 2 (Einsteins mass-energy relation)
RELATED QUESTIONS
An electron, a proton, an α-particle, and a hydrogen atom are moving with the same kinetic energy. The associated de Broglie wavelength will be longest for ______.
State the importance of Davisson and Germer experiment.
What is the speed of a proton having de Broglie wavelength of 0.08 Å?
Explain what you understand by the de Broglie wavelength of an electron. Will an electron at rest have an associated de Broglie wavelength? Justify your answer.
The de Broglie wavelengths associated with an electron and a proton are the same. What will be the ratio of (i) their momenta (ii) their kinetic energies?
Two particles have the same de Broglie wavelength and one is moving four times as fast as the other. If the slower particle is an α-particle, what are the possibilities for the other particle?
Find the ratio of the de Broglie wavelengths of an electron and a proton when both are moving with the (a) same speed, (b) the same kinetic energy, and (c) the same momentum. State which of the two will have a longer wavelength in each case.
According to De-Broglie, the waves are associated with ______
An electron is accelerated through a potential of 120 V. Find its de Broglie wavelength.
Calculate De Broglie's wavelength of the bullet moving with speed 90m/sec and having a mass of 5 gm.
The momentum of a photon of energy 1 MeV in kg m/s will be ______
The de Broglie wavelength associated with photon is, ____________.
According to de-Broglie hypothesis, the wavelength associated with moving electron of mass 'm' is 'λ e '· Using mass energy relation and Planck's quantum theory, the wavelength associated with photon is 'λ p '. If the energy (E) of electron and photon is same then relation between 'λ e ' and 'λ p ' is ______.
An electron of mass m and a photon have same energy E. The ratio of de-Broglie wavelengths associated with them is ( c being velocity of light) ______.
If the radius of the circular path and frequency of revolution of a particle of mass m are doubled, then the change in its kinetic energy will be (E i and E f are the initial and final kinetic energies of the particle respectively.)
A particle of charge q, mass m and energy E has de-Broglie wavelength `lambda.` For a particle of charge 2q, mass 2m and energy 2E, the de-Broglie wavelength is ____________.
How much energy is imparted to an electron so that its de-Broglie wavelength reduces from 10 -10 m to 0.5 × 10 -10 m? (E =energy of electron)
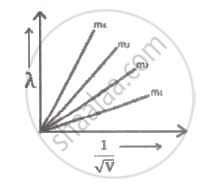
Graph shows the variation of de-Broglie wavelength `(lambda)` versus `1/sqrt"V"`, where 'V' is the accelerating potential for four particles carrying same charge but of masses m 1 , m 2 , m 3 , m 4 . Which particle has a smaller mass?
According to de-Broglie hypothesis, the ratio of wavelength of an electron and that of photon having same energy 'E' is (m = mass of electron, c = velocity of light) ____________.
Obtain an expression for de-Broglie wavelength of wave associated with material particles. The photoelectric work function for metal is 4.2 eV. Find the threshold wavelength.
The energy of an electron having de-Broglie wavelength `λ` is ______.
(h = Plank's constant, m = mass of electron)
An electron is accelerated through a potential difference of 100 volts. Calculate de-Broglie wavelength in nm.
Calculate the de Broglie wavelength associated with an electron moving with a speed of `5 xx 10^6` m/s. `(m_e = 9.1 xx 10^(-31)kg)`

- Maharashtra Board Question Bank with Solutions (Official)
- Balbharati Solutions (Maharashtra)
- Samacheer Kalvi Solutions (Tamil Nadu)
- NCERT Solutions
- RD Sharma Solutions
- RD Sharma Class 10 Solutions
- RD Sharma Class 9 Solutions
- Lakhmir Singh Solutions
- TS Grewal Solutions
- ICSE Class 10 Solutions
- Selina ICSE Concise Solutions
- Frank ICSE Solutions
- ML Aggarwal Solutions
- NCERT Solutions for Class 12 Maths
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for Class 12 Biology
- NCERT Solutions for Class 11 Maths
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 11 Biology
- NCERT Solutions for Class 10 Maths
- NCERT Solutions for Class 10 Science
- NCERT Solutions for Class 9 Maths
- NCERT Solutions for Class 9 Science
- CBSE Study Material
- Maharashtra State Board Study Material
- Tamil Nadu State Board Study Material
- CISCE ICSE / ISC Study Material
- Mumbai University Engineering Study Material
- CBSE Previous Year Question Paper With Solution for Class 12 Arts
- CBSE Previous Year Question Paper With Solution for Class 12 Commerce
- CBSE Previous Year Question Paper With Solution for Class 12 Science
- CBSE Previous Year Question Paper With Solution for Class 10
- Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts
- Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce
- Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science
- Maharashtra State Board Previous Year Question Paper With Solution for Class 10
- CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts
- CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce
- CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science
- CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10
- Entrance Exams
- Video Tutorials
- Question Papers
- Question Bank Solutions
- Question Search (beta)
- More Quick Links
- Privacy Policy
- Terms and Conditions
- Shaalaa App
- Ad-free Subscriptions
Select a course
- Class 1 - 4
- Class 5 - 8
- Class 9 - 10
- Class 11 - 12
- Search by Text or Image
- Textbook Solutions
- Study Material
- Remove All Ads
- Change mode
De Broglie's Hypothesis ( AQA A Level Physics )
Revision note.

De Broglie's Hypothesis of Wave-Particle Duality
What was debroglie's hypothesis.
- Louis DeBroglie hypothesised that all particles can behave both like waves and like particles, following Einstein's work with photons
- By equating two equations from Einstein, he derived an equation for the momentum of a photon:
- Where h is Planck's constant, c is the speed of light, m is mass, λ is wavelength and f is frequency
- mc is the momentum, p , of a photon - DeBroglie extended this idea to particles with mass to obtain the relation you should recall from Particles & Radiation:
Finding the Wavelength of Accelerated Particles
- Finding their momentum directly is difficult, but recall from The Discovery of the Electron that the work done on an electron by an electric field ( eV ) is equal to its kinetic energy - this can be used to find the electron's speed:
- This can be substituted into the momentum term in DeBroglie's hypothesis to then find wavelength:
- The wavelength of the electron depends on the work done on it by the electric field, eV
- From this equation, as eV increases, λ decreases
- When the electron is accelerated to a higher speed , its DeBroglie wavelength decreases
Worked example
An electron is accelerated through an electric field and is found to have a DeBroglie wavelength of λ . The potential difference across the electric field then increases by a factor of 25. Write the new wavelength of the electron in terms of λ .
Step 1: Write out the equation for an accelerated particle's wavelength from your data and formulae sheet:
- The wavelength of an accelerated particle is:
Step 2: Label the new wavelength and substitute the new potential difference:
- Now we will manipulate this expression until we can pull out the original expression for λ :
- Therefore the new wavelength is:
- This checks out with common sense - the particle is moving faster under a stronger potential difference so, as was mentioned above, its new wavelength should be smaller
This equation requires some confidence in algebra involving square roots. Remembering that you can combine square roots when multiplying or combining will help a great deal:
You've read 0 of your 10 free revision notes
Get unlimited access.
to absolutely everything:
- Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Exams
the (exam) results speak for themselves:
Did this page help you?
Author: Dan MG
Dan graduated with a First-class Masters degree in Physics at Durham University, specialising in cell membrane biophysics. After being awarded an Institute of Physics Teacher Training Scholarship, Dan taught physics in secondary schools in the North of England before moving to SME. Here, he carries on his passion for writing enjoyable physics questions and helping young people to love physics.
Things you buy through our links may earn Vox Media a commission.
The Asteroid-in-Spring Hypothesis
Two paleontologists have turned on each other, each claiming to have found new evidence about the worst day on earth..

In August 2017, a bubbly Dutch pink-haired 28-year-old graduate student flew from Amsterdam to South Dakota, where empty fields rolled wide before her, towns of a hundred people and a single church. “You a celebrity or somethin’?” a man had said last time she was in the area, picking up a can of Monster at a truck stop. “Not to my knowledge,” she said. Melanie During had never been to New York, or Los Angeles, or Chicago, but she was already familiar with this particular landscape, dense with buried bone.
Also in town was a 70-year-old Dutch paleontologist named Jan Smit, a man she got to know the day she dissected an ostrich in his kitchen. With him was a stranger, a 35-year-old American graduate student. The three of them drove to what During casually calls “a triceratops mass grave,” at which point, to her surprise, Smit left.
For the next few days, it would be the two students and whatever the ground gave up. Her companion drove a 4Runner under the arc of a giant sky feathered with clouds, through panoramic prairie, fields of buffalo, mud buttes rising against the horizon. He pulled off a gravel road and right onto a ranch.
They got out of the truck. With each step, dry grass crunched under their feet and grasshoppers sprang in all directions. Through the knee-high thistle, it was hard to judge where each footfall would land. The grass stopped, and the earth dipped into a gnarled mass of rock and clay. The land was strange, full of odd textures, scaly in spots, darkly reptilian, and blanched out in others. He was proud of the place. While the site was not technically his property, it was spiritually his, shaped and carved and loved by him, and During was there with his permission. He called it Tanis, so everyone else did too.
Robert DePalma is solid and dark and affable and shares with During a certain kind of rough history, but he is, according to Smit, During’s foil. When During looks at a bone, she sees a chemical matrix waiting to be investigated. She sees an opportunity to extract information. When DePalma looks at a bone, he sees a narrative. He tells stories about the bones, some of them true.
“He is secretive,” says Smit. “Melanie cannot be more the opposite. She’s in all the social media. She makes herself known.” One way that she makes herself known is by posting pictures of herself cradling a model of a baby T. rex like a prehistoric Pietà (“sweet baby Jesus Rex,” she calls it on Instagram) or riding a giant reconstructed dinosaur like a cowboy atop a horse. “I have had a great deal of criticism directed at my work,” she once said, “which was actually criticism of my flamboyant personality, my big mouth.”
During would have only a handful of days at Tanis, but they were days unlike any she had seen before. On a normal dig, it is typical to find nothing of note for long stretches. “You pee in the bushes,” she says, “you get chased by snakes, you find no fossils.” Here, she would brush away clay and come upon a new texture and color — a precious fossil fish. The first sighting gave her goose bumps. Blowing on the fossil would have been too violent an approach; she might waft away the very thing she sought. She would begin to gently carve out sediment around the fossil, but there, astonishingly, would be another one. “A luxury problem,” she calls it, “stumbling on all these other fishes in the way.”
The exact location of the site remains secret, vulnerable as it is to poachers and rival paleontologists. Almost no one knew about the place in 2017, but a few years later there would be magazine features, multiple documentaries, conference presentations and journal papers. David Attenborough, science’s most beloved narrator, would tell his audience of a “truly extraordinary dig site” against a backdrop of an asteroid hurtling through space. “No one has ever found the fossil of a dinosaur that they know for certain died as a result of the impact,” Attenborough would say. “This place might hold evidence of one of the most dramatic events in all the four-and-a-half-billion-year history of our planet.” The New Yorker would publish a feature centering DePalma as a controversial young scientist with a major discovery.
During and DePalma both believed the fish at Tanis died in a violent flood less than an hour after an asteroid hit the Earth, killing off the non-avian dinosaurs. This is why they found fish pointing in both directions, their bodies broken and speared with debris; like a pool in an earthquake, the river rocked back and forth, throwing sea life upward to land wherever it might fall and be entombed in layers of mud. “A car crash frozen in place,” as During puts it, a freeze-frame from 66 million years back. They would both converge on the same mystery, tunneling toward greater precision: In what season had the asteroid struck?
DePalma had set up two tents for them off-site, close to town, where they would sleep and have downtime, though downtime is not something During has ever really appreciated (“I don’t sit down and watch things” is her take on TV). All day long, in temperatures inching toward 100, they crouched over the delicate petrified remains of sturgeon and paddlefish, trying to position their feet such that they could draw remnants from sediment without crushing something that had survived 66 million years intact. They smacked at biting flies. They dug through hot days and slept in tents that failed to keep out the rain.
“Personality-wise, and this is really not about personalities … I mean, I don’t want to make it about personalities,” DePalma told me years later, resisting further specificity. “Personality-wise, she wasn’t necessarily someone I would normally be friends with.”
“I don’t want to judge people for how they come across on a personal level,” During told me, declining to elaborate, “but there were moments where I thought, It would’ve been very helpful if I could have just had a word with someone else. ”
None of what follows will make sense absent a single social fact: The field of paleontology is mean. It has always been mean. It is, in the words of Uppsala University professor Per Ahlberg, “a honeypot of narcissists.” It is “a snake pit of personality disorders.” “An especially nasty area of academia,” the Field Museum’s Jingmai O’Connor calls it. Among the subfields, nastiness correlates with the size and carnivorousness of the creature under study, the comity possible among those who study ammonites being unlikely among those who study T. rex. A “social experimenter with a penchant for sadism” is how his biographer describes Sir Richard Owen, the man who coined the term dinosaur. The first two famous American paleontologists, the prickly academic Othniel Marsh and the gentleman naturalist Edward Cope, savaged each other in print, hired spies and counterspies, destroyed fossils, and generally worked harder to humiliate each other than to describe the boxes and boxes and boxes of remains they pulled from the extraordinarily rich fossil beds of the American West.
It would take years for the ten days During and DePalma spent together to spin into a scandal that consumed both of them. She would accuse him of research misconduct and fabricating data. He would accuse her of plagiarism and defamation. He would lose weight and have flashbacks to childhood bullies; stress would pose a risk to her first pregnancy. Disaster struck one day in the spring, they both decided in the end, and transformed everything that came after.

We don’t know how to read history in water; we know how to read it in bone. The West, in particular a 500-kilometer stretch of rock known as the Hell Creek Formation, is an ideal place to preserve fossils because it is given to collect sediment and it is dry. A bad place would be the tropics, where a dead animal is likely to be eaten before it can be buried. A bad place would be a mountain summit, where a skeleton would be swept off on the wind. A truly terrible place would be the waterlogged nation of the Netherlands, where amid all that peat and loam and sand only a single dinosaur species has been discovered and described. It is a strange place in which to be a paleontologist, but it is where Melanie During was born in 1989, in a land unsuited to fossilization to parents unsuited to parenting.
The little home in Langedijk was not one in which scientific insights seemed likely to develop. Melanie’s father was absent, and, according to her, “a twat.” Her mother was ADHD, autistic, and, by her own description, inadequate. “I was not fit to do the job alone,” she says, “and I was alone,” though she took pride in being a child with her four children, singing and painting and presenting them with great bags of clay they could, together, manifest into shape. Melanie’s ambition stood out to her family in North Holland. “She loves the spotlight,” says her sister, a trait perhaps more befitting the United States, the country that held all the treasure and the trouble to come.
The Dutch school system makes distinctions early, and 12-year-old Melanie was placed on the least intellectual of three tracks, headed not for university but for trade school. Did Melanie want to be a plumber or a hairdresser? No one in her family had been to university. The decision to place a student on such a path is made, sometimes, with the knowledge that not all parents are capable of helping with rigorous schoolwork. In Melanie’s telling, her mother forced the older sister out of the house, sent a younger sister to live with her father, and often disappeared herself, leaving 16-year-old Melanie alone to care for an autistic 6-year-old brother. She shoplifted soap and cheese and maxi pads. She stopped speaking for a time. She went into foster care.
After school, Melanie picked tulip bulbs, delivered newspapers, cut roses, waited tables. Social life was a struggle. “She had a feeling,” according to her mother, “that people didn’t like her.” When Melanie was about 15, her history teacher was concerned about the isolated, chubby girl who seemed to have surrendered the very idea of fitting in. “I was bullied, too,” the teacher told me. In front of the class, the teacher crumpled a piece of paper into a ball and flattened it back out. This, she said, is what happens when you bully someone and then apologize. The paper is never quite right again.
Melanie told the history teacher that she would like to go to university but doubted this would be possible. The history teacher devised a complicated plan of tests and classes. Melanie followed the plan and thrived. Having had to take care of herself for much of her childhood, find a bed, find dinner, she was too independent for her foster parents and successfully petitioned to be emancipated at the age of 17.
By 2017, During was a master’s student in earth sciences at Amsterdam’s Vrije Universiteit working on her thesis. She was examining rocks from a Dutch quarry using a method called stable-isotope analysis. To her great disappointment, she could not find anything earth-shattering to share. “All I could say,” she recalls, “was it was very hot and it was very saline.”
Avoiding work, During attended a lecture by Jan Smit on the occasion of his receiving the Netherlands’ highest award for earth sciences. Smit was talking about his trip to an extraordinary new paleontological site in North Dakota. The site was called Tanis. It occurred to her that if one performed stable-isotope analysis on the bones of the fish at Tanis, one could discover something about the moment in which they had died. Maybe she could find something more to say than: It was hot and salty. She began composing an email to Smit on her phone, right there in her seat, during his talk.
Smit already knew of this teaching assistant. She was the one who had asked him whether he had a pot large enough in which to boil an ostrich after she had procured an ostrich carcass and decided to dissect it for fun. “Somebody who wants to do something like that,” says Smit, “that’s a girl I like, who’s not afraid to do the experiment, is not afraid to make her hands dirty.” Smit told DePalma he had a student familiar with stable-isotope analysis, a subject in which DePalma had no particular training, and DePalma said she could visit. During had lots of ideas and no money. All her travel-grant applications were denied. Smit lent her the money to go to North Dakota.

The knowledge that an asteroid killed the dinosaurs is knowledge recently acquired; most paleontologists working today did not grow up learning that a rock six miles wide slammed into Earth and ended T. rex. The “Alvarez Hypothesis” was published in 1980, shortly after scientists found a layer of iridium locked in rock the world over and surmised that it could only have come from space. It is called the Alvarez Hypothesis and not the Smit Hypothesis because Luis Alvarez and his son Walter got their paper out before Jan Smit. As Peter Brannen put it in his excellent The Ends of the World, “The Alvarezes published first and were immortalized. Jan Smit doesn’t have a Wikipedia page.” Smit does seem to have acquired a page since the publication of Brannen’s book; under the heading “Known For,” the page reads: ALVAREZ HYPOTHESIS.
This is not to say that the idea was readily accepted; it sounded ridiculous, bombastic, childlike in its sudden simplicity, and the Alvarezes spent the ’80s arguing against those who attributed the end of the Cretaceous to excitable Indian volcanoes. In order to support his theory about a space rock with the force of 4.5 billion atomic bombs killing off giant reptiles, Alvarez had to find a crater the size of Connecticut. He looked in Iowa. He looked in the ocean; he was pretty sure it was in the ocean. He could not find it. If you’re so sure a massive asteroid felled the dinosaurs, the volcanists asked, why can’t you find this giant gaping hole? How hard could that even be? Conferences were held and concluded, craterless.
In fact, the crater had already been found, in the Yucatán, by a gregarious, eccentric oil-seeking PEMEX geophysicist named Glen Penfield. Penfield noted anomalies in a magnetic field, charted it with paper and a pencil, found a circle the size of Connecticut, and surmised, before anyone else, that he had found the crater in question. He called Walter Alvarez, left a message, and got no response. (“A mediocre geologist,” Penfield calls him now.) He tried telling NASA and was rebuffed. He had been trying to share the news about it for a decade, but the attitude, according to Penfield, was “This kid doesn’t even have a doctorate” and it’s “not worth talking to some oil guy.” He spent a considerable amount of time, he told me, depressed that no one would hear him, not even a mediocre geologist whose reputation hinged on this very information. He named the crater Chicxulub specifically “to give the academics and NASA naysayers a challenging time pronouncing it after a decade of their dismissals.” Yucatán Crater would have been too easy for them.
It was before grass, before beans, before the 24-hour day. In film, this has been represented as a man gazing into the sky as a rock floats into his field of vision, but this is a confusion born of our inability to understand speed and scale. You would have not had a moment to turn toward the sky; as Brannen explains, the rock, six miles long, shot from the height of an airborne 747 to the ground in .3 seconds and continued onward toward the center of the Earth, 12 miles into crust. In its wake it left a vacuum that sucked in shattered and melted masses of this planet and shot them into space. The shock traveled through the oceans; tsunamis hundreds of feet tall rose skyward. Bits of earth, ejected into space, fell back through our atmosphere on fire, a rain of flame. The surface of the planet grew hot as an oven set to broil. T. rex , triceratops: These were not creatures designed to hide. A layer of iridium settled over the globe, to be buried by millions of years of sediment and discovered by Jan Smit 66 million years and a few weeks too late. In the same layer, known as the K-Pg Boundary, geologists would find tektites — bits of earth that shot into space when the asteroid hit, turned glassy with the heat of the atmosphere, and fell back to the surface. Smit called the smallest ones spherules. That DePalma claims to have found spherules all over Tanis is some of the strongest evidence for the site being a historical record of impact. Spherules appear in sediment like gnarled bits of clay. They look sometimes like BBs; when they’ve fused together, they look like Nerds.
DePalma did not find Tanis. The site was found on a piece of private property by two prospectors who had a modest business bringing fossil enthusiasts to the Dakotas. A good day for Steve Nicklas or Rob Sula, who paid ranchers for access to the site, would mean coming upon a fish tooth or a fragment of fish spine. Most fossil finds are a pile of puzzle pieces. On a single day at the ranch in 2008, they came upon a fragment of a sturgeon skull, brushed off some sediment, and found an entire articulated sturgeon. “We knew it was important right then,” says Sula. “Three-dimensional. There were scales on this thing. It was obviously super-gentle preservation.” Nicklas and Sula began to dig it out with a scalpel. “Once we uncovered the first fish,” he says, “there was another one.” There were, in fact, dozens and dozens of 66-million-year-old fish, “stacked up like cordwood, multiple matrices with multiple densities.” It was difficult, thrilling work. “The single fully articulated sturgeon was the first time I’d ever seen anything like that,” says Sula, “let alone stacks of them!”
At least this much is indisputable: Nicklas and Sula located a Lagerstätte : a site of extraordinary preservation. They sent some fish to the University of Chicago’s Field Museum and called in an academic to assess what they had found. Nicklas called his friend, a dinosaur prospector named Ron Frithiof. Nicklas and Frithiof had something in common: They liked underdogs and distrusted institutions. Frithiof, in particular, feels “most academics are arrogant assholes.” But he knew one he liked, an academic who seemed like “a regular person.” His name was Robert DePalma, and he was just a kid, really — a graduate student in paleontology at the University of Kansas.
In Nicklas and Sula’s view, they had done DePalma a favor, and they continued to share the site with him for seven years. They were thus shocked, in 2019, to find themselves described in his celebratory New Yorker feature as private collectors who thought the site was “a bust” and so cluelessly passed it on to DePalma, who deemed them irresponsible for failing to properly excavate a dinosaur’s hip bone.
“That put me in a super-dark place,” Sula told me. “It was astounding how in a couple of sentences, they managed to marginalize us and omit us from history.”
Sula was upset enough to call The New Yorker and complain. Soon after that, a woman who helped run the ranch told Sula and Nicklas they weren’t welcome back; the family felt they weren’t following rules regarding fire safety. Sula was devastated. “It was a personal thing,” he says. “I loved that place. It was beautiful, and it was part of who I was.”
He still loves the place, but he’s not convinced it’s a record of the day the dinosaurs died. He thinks it might be what he always thought it was, a “fish site” with spectacular preservation. A lot of people do.

It remains a matter of dispute when and where and with what antecedent Melanie During came up with the idea for determining the season the asteroid killed the dinosaurs. But the idea was this: Sturgeon bones grow like tree rings, and the bone cells grow thickest in summer, when food is most plentiful. A slice of bone, then, should reveal a succession of seasons. Months of plenty would be thicker, as the fish grew fat on plankton. The outermost bone, the last stage of bone growth before the asteroid, should reveal the season of death.
During had a single year to finish her master’s thesis after her 2017 visit to Tanis, and she did not have the money to extend the time. If she did not finish the thesis, she would not graduate and would not be able to apply to Ph.D. programs. “When people tell you ‘Relax,’ I’m like, What’s that? ” she told me. “What do you mean? Do I sit down and do nothing?”
She was not an expert in fossil fish, or histology, or the Late Cretaceous; she had learned isotope-analysis methodology only the previous year from her adviser, Jeroen van der Lubbe. She was a few years out of undergrad — not even a doctoral student. The demise of the non-avian dinosaurs is perhaps the best-known division in all of paleontology, a historical moment that reaches beyond the classroom into childhood nurseries. It is constituent of the way our culture structures the history of the universe, basic to the way we have come to categorize time itself. For a Dutch 20-something to believe she would contribute new information regarding this moment in a master’s thesis was potentially delusional, but During felt the fish from Tanis contained long-hidden knowledge the right methods might evoke.
Her first attempt to measure the growth lines was a failure, four months lost on a faulty technique. “It was a waste of my time to continue,” she says. “I don’t cry over spilled milk.” She had to beg and wheedle for equipment; she was expert in very little but unafraid to ask for help. In order to understand the chemical composition of her samples, she needed to understand what was the original bone and what was a chemical artifact of the process of fossilization. To do that required a Micro XRF spectrometer, which she did not have, but a friend in Brussels had access to one; she drove to Brussels, and there, for the first time, she clearly saw the growth lines in slices of bone.
During’s naïveté would be, over and over, her strength. She intended to measure the isotopic ratio between two molecules at different points in the growth lines. In a university building that still stands only because there is a nuclear reactor in the basement, in a room with a window taped against the wind, During positioned and repositioned a tiny slice of fish bone in a micromill. She came into the little room before dawn and left when it was dark. “You’re still here,” the lab manager, Suzan Warmerdam-Verdegaal, would say on her way out of the building. The mill produced specks of fossil, smaller than a grain of sand. One day, after she had labored for many hours to collect a few samples, a colleague opened the door. The samples wafted into the room, lost to her. That day, she did cry.
A bit of luck: At a dinner for During’s partner on the occasion of his doctoral defense, another scientist with connections to the European Synchrotron Facility offered her some time there — a valuable and vanishingly rare opportunity for a young academic. She told DePalma, and he mailed her a fish skull. She brought the skull to an 844-meter tunnel for speeding electrons, and shot through it beams of light a million times brighter than the sun. The scan revealed the interior and inside the gills, incredibly, little bits of trapped clay: spherules that had traveled from the Yucatán to space and fallen back to Earth to be inhaled by a doomed paddlefish.
Back in Amsterdam, During worked at the mill, but the bits of precious dust failed to accumulate in the way she had hoped. It was April; she was a student only until late August. She didn’t have money to continue her studies. It was Warmerdam-Verdegaal, the lab manager, who devised a solution. Even with very few samples, the isotopic analysis could be made to work with a cold trap, a piece of equipment the lab had but that no one in the lab had ever used for this purpose. She called During one day in May. “We have results to the end,” she said. During screamed and biked to the lab. After days of plotting graphs, she had it: These fish, the isotopic results confirmed, had died in spring.
She defended her master’s thesis in a dress she had sewn herself, in a print with triceratops and ankylosauruses marching across palm trees. It was late August, and the department was mostly empty; her adviser walked through the corridor pulling people into her defense so she would have someone to defend to. She had not only found seasonality but helped develop a new way to combine osteohistology and isotopic dating. Twelve years earlier, she had been a kid in foster care.
The thesis won the Dutch Escher Prize for the most outstanding master’s thesis in earth sciences. She sent it to DePalma and his co-authors, asking that they keep quiet because she planned on publishing the work later, with DePalma as a co-author, since he controlled the site and provided the fish.
Not long after, Smit received a lengthy email from DePalma. “I was naturally surprised to see that Melanie’s entire thesis, even the title, apparently became refocused specifically on determining the time of year,” he wrote. “There are serious ethical concerns here.” He claimed that the subject and methods had been “extracted directly” from the ongoing work of his research team. The idea for using histology to determine time of year, he said, originated with his colleague Gregory Erickson; she “explored a concept, technique, and research goals that were already being written up by us.” He wrote in a tone of deep agitation unsuccessfully masked by politesse. “I hope all of this gets sorted out in the best way possible” he concluded, “and I now have some valuable insight into what it is like to work with Melanie.”
Smit related the email to During, suggesting that it was all a misunderstanding soon to be resolved. But During was devastated. Now, she would never publish a paper on her work because the results would be contested, the path to a Ph.D. less clear. Now, someone else, someone equally ambitious and working against her, had the method she and her advisers had developed. I sent him, she thought, a fucking cookbook.
I get these all the time from crazy people,” says Kirk Johnson, the director of the Smithsonian Museum of Natural History. “People are always saying, ‘I discovered the Ark of the Covenant.’” He received such an email in 2012 from Robert DePalma when DePalma was a 30-year-old graduate student. “It was a very weird kind of secretive, extraordinarily hyperbolic email that said, ‘Look, I’ve made the most amazing discovery in the history of the planet. I found all this stuff that’ll change the way we view science in the world.’” This guy’s nuts, Johnson thought, and did not respond. The next time he came across DePalma was at a gathering of paleontologists and ranchers in Montana in 2016. DePalma clicked through pictures of himself and his old-style tent. He was writing by hand, on a table strewn with a pipe and a porcelain teacup. “With a lantern!” Johnson recalls. “Nobody uses a lantern!” DePalma described what he had found in Hell Creek. “He said, ‘I actually found a complete gecko in amber.’” In the published literature to date, no one has ever found a large section of amber in Hell Creek; one tends to find tiny pieces and in them microfossils. “To find an entire gecko in amber is, like, the holy grail,” says Johnson. This would have been a major, history-making discovery. “And he flashes this fuzzy image up on the screen and then takes it off, and then he leaves. And we’re all like, What the fuck was that? ”
DePalma says he showed crisp slides of a partial gecko in a peanut-size nub of amber (and provided clear slides to New York ) but does not dispute the lantern. Cosplay is a word that comes up often in conversations about him; he favors an impractical leather hat, suspenders, dramatic looks into the distance. He digs with a pick possibly owned by an associate of Othniel Marsh, the original rapacious paleontologist, though he prefers the Quaker Cope. If many people, in discussing Robert DePalma’s sartorial choices, will feel the need to explain that Indiana Jones was not even a paleontologist (he was an archaeologist), it is worth noting that the field of paleontology has always had an element of costume.
While a grad student at the University of Kansas, DePalma led a research team that unearthed what he called Dakota-raptor, a 17-foot flightless winged carnivore he described to The Guardian as “the Ferrari of competitors” and “the most lethal animal you can possibly imagine into the paleoecology of that time period.” In the pictures he provided to the paper he wears a suit vest and holds a shovel like a 19th-century showman. Pretty much as soon as DePalma unveiled it, Dakotaraptor was controversial. Other researchers pointed out that the bones he had assembled contained a turtle shell. Such mistakes are easy to make; Marsh made fun of Cope mercilessly and repeatedly for attaching a plesiosaur’s skull to the end of its tail. DePalma, always sensitive to criticism, was attacked online for his possibly chimerical discovery. This was part of the reason Frithiof decided to give DePalma the tip about the “fish site.” He thought DePalma had been badly treated. He thought the kid deserved a break.
In the fall of 2016, DePalma publicly announced, at the annual Geological Society of America meeting, that he had found a mass grave full of spherules. The site had been created, he said, from a giant, deadly flood minutes to hours after the asteroid hit. It was the scene of our greatest natural disaster, hidden from us until now. Audible gasps rose from the crowd.
“I was incredulous,” says Johnson, who has been studying Hell Creek for decades, discovered in 1987 the first K-Pg Boundary site in North Dakota, wrote a book about Hell Creek, found a post-asteroid boundary layer with spherules fewer than two miles from Tanis, and is generally the leading expert on the geology and paleobiology of the region. After the conference, Johnson continued to hear of claims DePalma was making. “He proceeded to list a bunch of things that were outrageous. Like, ‘I found an egg of a pterosaur; I found dinosaur feathers.’”
On a trip to Hell Creek in 2016, when DePalma had the lease on Tanis but had not yet made his dramatic claims about it, Johnson and fellow paleontologist Tyler Lyson were engaged in a project to map the K-Pg Boundary along the entirety of Hell Creek. “I’ve been thinking about this sort of stuff basically my whole life,” says Lyson, a curator at the Denver Museum of Nature and Science who grew up fewer than 20 miles from Tanis. They ran into Sula and Nicklas, who invited them to check out the “fish site.” Lyson and Johnson measured its elevation. After the GSA talk, Lyson went back to his notes. “And I’m like, Dang, it’s much, much lower than you would expect. ” At first glance, at least, one would expect a site at that elevation would be older than 66 million years.
Even before his announcement at the GSA, DePalma began to bring journalists and scientists to Tanis. As a student working toward his Ph.D., DePalma contacted none other than Walter Alvarez, the man most associated with the asteroid itself. Alvarez visited the site, was convinced, and became a supporter. DePalma also contacted the novelist Douglas Preston, who visited the site in 2013 and wrote the article that would eventually appear in The New Yorker. Preston’s account is full of finds as shocking and world-shattering as the gecko in amber: a fossil feather, a dinosaur egg, and the remains of a mammal inside the very place it would have hid during the impact.
“Is that a burrow?” Preston asks in the piece.
“You’re darn right it is,” says DePalma.
Paleontologists were skeptical at the time, but their quotes to the media were measured: Let’s wait and see what he publishes. They would judge the findings by the peer-reviewed papers to come. Tyler Lyson did his own investigating. After the explosive GSA talk, Lyson got a three-by-two-foot block of Tanis dirt from Rob Sula, who had removed it before ceding control to DePalma. Lyson hadn’t opened it, but after reading the New Yorker article he prepped it in his museum in Denver. He found fish. He did not find spherules inside the fish. He did not find, in the dirt around those fish, a single spherule. His suspicion grew.
There have been, in the decade since DePalma claimed to have found a dinosaur feather, a mammal burrow, and a pterosaur egg, no resultant publications on these particular finds. Academic paleontologists must keep their fossils publicly accessible; they might be kept at the Field Museum or the Denver Museum of Nature and Science. They are given accession numbers, attached to a museum or university, which researchers use to refer to and request them, as with library books. DePalma is associated with the Palm Beach Museum of Natural History, which is a storefront in a mall, but his finds are not displayed there. When Frost Museum paleontologist Cary Woodruff called the Palm Beach Museum of Natural History asking for a cast of a Dakotaraptor claw DePalma had found, he was put off for about three years by an “exasperated and apologetic” museum employee. Finally, he says he was told by the museum that DePalma kept the bones in his home, in a safe. “As paleontologists,” Woodruff says, incredulous, “we do not store fossils at our house.” (DePalma, asked if he has kept bones at his home, suggests the employee was joking.) When I asked about the staggering fossils described in The New Yorker, DePalma said they were at Florida Atlantic University, where he teaches, and which did not respond to requests for comment.
Paleontologists who might have felt cautiously skeptical in 2019 are now openly and vocally baffled. “No one’s ever published a dinosaur feather from anywhere in North America,” says Johnson. “He has dinosaur feathers. Why didn’t he show us a picture of them? That’d be the cover of Nature. If he had a pterosaur egg, why didn’t he publish? These would be amazing, major discoveries if they were true. “
In the New Yorker feature, DePalma unwraps “a sixteen-inch fossil feather” and holds it “in his palms like a piece of Lalique glass.” “I mean this is just some cartoon, made-up idea of what paleontology is like,” says Jingmai O’Conner, the Field Museum curator who spent ten years working in China, where most fossil feathers have been found. “Fossil feathers don’t preserve three dimensionally in ways that you could hold them, unless they’re in amber. But then you’re actually holding a chunk of amber.”
Greg Wilson Mantilla of the University of Washington is the foremost expert on Late Cretaceous mammals of Hell Creek, a subject he has studied intensively for 25 years. In a day of digging, he says, “you might find a molar of a mammal and know that the mammal was contemporaneous with all the other little pieces that you’re finding. At best we’ll find a jaw that has a couple of teeth in it. That’s a red-letter day.” DePalma has claimed to have not only found a mammal jaw and shoulder bone, but to have found them inside an intact mammal burrow that crosses the K-Pg Boundary. Wilson Mantilla, having devoted his life to this narrow realm of study, would very much like to see the fossils, but his extensive efforts to coordinate with DePalma have not borne out. When they met at a conference, Wilson Mantilla says, DePalma was solicitous and admiring. “He said, ‘I’d love to get you out to the site and, you know, get you the specimen to look at.’ And so I followed up with him. But this began a series of, um, basically exchanges that never amounted to anything. He either didn’t respond to me or promised that I could come out to the field site, just contact him when I get out to Montana, and then I’d link up with him in North Dakota. And it just — everything always fell through.”
The questions swirling around DePalma necessarily implicate his most fundamental claim: the legitimacy of Tanis as the single site known to record the last moments of the Cretaceous. In 2019, DePalma published for the first time on Tanis, in the journal PNAS. This paper, which made the case that Tanis records a brief time after the asteroid hit but did not reference the specific fossils mentioned earlier, was greeted skeptically, though both Jan Smit and Walter Alvarez were co-authors. “I’m not a great geologist,” Woodruff told me, “but Ray Charles could see the geology didn’t make sense the way they described it.” “No one I’ve talked to who’s not involved in the project,” says University of Alabama geologist Tom Tobin, “was convinced by that first paper.”
Those who buy into Tanis tend to focus on the considerable authority of Smit and Alvarez, giants in the field who stand by DePalma’s claims. Smit says he has seen the Tanis spherules, right there in the dirt, and the dinosaur feather in person. But DePalma’s reputation is such that even this does not settle the question. “You can buy spherules at the Tucson mineral show,” one paleontologist told me.
The person capable of making the best case for Tanis is neither Smit nor Alvarez but someone who has accused DePalma, publicly, of faking data. “I will admit,” Woodruff says, “I didn’t think in my professional opinion that any of Tanis was legitimate.” And yet Woodruff is less sure today because he trusts Melanie During, whose work stands, ironically, as the site’s best defense. In During’s synchrotron scan, her fish head appears to have, lodged in the gills, Nerds-like objects.
“How the hell do you plant them in a fish?” says Woodruff. “Like, I don’t think that can really happen. Melanie’s stuff is the only time that I’ve been like What if? and Huh! ”

DePalma has been working Tanis since 2012, talking about seasonality since at least 2013, and first encountered the concept of using histology to determine the season of the asteroid in conversation with paleobiologist Gregory Erickson in 2016. When During visited a year later, DePalma says he provided her with orientation materials that read “The Fall of the Cretaceous: Month Scale Timing of the Cretaceous Apocalypse,” predicted a “late spring to late summer” mass death, and mentioned “histological and isotopic results,” though it had equal emphasis on locating the flora that died at the same time. DePalma requires an “application” for visiting Tanis, which he says is typical but a number of other paleontologists say they have never before encountered. On her application, During indicated she would be investigating the fish’s various injuries, a fact DePalma noted when he expressed surprise that her thesis, which she sent him only after it was completed, was fully oriented toward pinpointing the season at spring.
“After reading your whole email, I can imagine you’re upset!” wrote Smit in response to DePalma. “Apologies for that, but to tell you the truth, we (Melanie’s supervisors) were also surprised by her last moment change of title. She clearly was carried away with the possibilities of the scans she made!”
In DePalma’s view and that of his colleagues, she had lied about her intentions, come to his site at his invitation, stayed in his tent, investigated fish that he packed up and sent her, concealed the subject of her thesis, and stolen his idea. This was outrageous to him, but it was something he could tolerate so long as the results were confined to an unpublished thesis. That she would publish them as first author was intolerable. “It was his idea,” says Pete Larson, the president of the Black Hills Institute and a co-author on DePalma’s paper. “It wasn’t her idea. That’s the thing that really kind of irks me — she did not come up with that idea on her own.”
Smit knew finding asteroid seasonality was DePalma’s plan, but he also knew the histological isotopic techniques had been developed, through trial and error, by During and others in the lab at Amsterdam. He suggested they write two separate papers and merge them into one. DePalma said he would send his manuscript; it was, he said, almost ready. It never, according to Smit, arrived. DePalma maintains that During never accepted the idea of merged manuscripts.
“Maybe I didn’t want to work with someone who accused me of theft,” During says to this. She concedes that the idea was DePalma’s. In her telling, it was he who suggested she search out the season of the asteroid in the ancient bones, and he was supportive of her work until he suddenly became hostile. Robert told her to “fill in whatever” on the application, she says, so she gestured generally toward her research interests.
In 2019 During applied for jobs and received a string of rejections. Eventually she found a role with Per Ahlberg at Uppsala University in Sweden. Ahlberg hired her, in part, because he thought she had been badly treated. He thought she deserved a break.
DePalma was a threat to During’s reputation, disastrous on a job market with many Ph.D.’s and few jobs, and by 2020 this was a massive source of stress. When she discovered that she was pregnant, she and her partner decided that she should step away from the issue of the unpublished Tanis research. She sewed a felt dinosaur mobile for the son she would soon have. She painted three marine reptiles underwater. She painted a long-necked dinosaur cradling a baby and a T. rex in the sun. Along with all the dinosaurs, she painted exactly one rocket ship. (“He doesn’t have to be a paleontologist,” she told me.) In between being induced and giving birth, she painted three more paintings of birds. The baby would be named Odin, an anagram of dino.
In 2021, Ahlberg encouraged During to submit the paper for publication despite the friction with DePalma. She emailed him to ask whether he wanted to be listed as an author, and weeks later, when he still had not responded, deleted his name.
“She waited for two years before she published anything,” Smit says. “I mean, two years is a long time in a scientist’s life.”
When he heard During was about to publish, a co-author of his told me, DePalma began to rush. His own paper would try to prove the asteroid-in-spring hypothesis through a number of separate overlapping methods, including showing what flora were found in the area, so as to leave little room for criticism.
During was 32 when, six months later, she read the email granting her paper a preliminary acceptance in Nature. Her master’s thesis — not even Ph.D. work — would be published in the world’s most prestigious scientific journal. It was too much to lose. She thought, Something will go wrong. It will never happen. Instead of celebrating, she focused on cleaning her house; her sister, terribly allergic to dust, was coming for a visit.
Two days later, as she was finishing up work, late to pick up her son from day care and her sister from the airport, her phone lit up with a text from Smit. “I should not have clicked the link,” she says, but she did click the link. DePalma had published his paper in the journal Scientific Reports. He was first. He was Alvarez; she was Smit. She drove through a blizzard with her son in the back of the car, working hard to see the road. Okay, she thought. I’m not going to publish. She ran out of wiper fluid, and the wipers could not keep up with the slush. He scooped me. It’s done.
As a child, Robert was, he says, “absolutely mercilessly bullied.” He spent a lot of time at home, though that entailed dealing with his parents’ divorce and custody battles. Alone in his room, he constructed hobby-store dioramas and small models of long-extinct beings. He adored cuckoo clocks; either he would be a cuckoo-clock-maker, he reasoned, or a paleontologist. Something in his body was conversant with matter, with bones. His father, an endodontist, operated on teeth for a living. His great-uncle, an orthopedic surgeon, wrote books with titles such as Diseases of the Knee. Robert did not care for sports, and the other children evidently did not care for clockwork. “I did not get a moment of peace from them,” he says. “I couldn’t even eat lunch.” He was born and raised in Florida, a barely surfaced peninsula in which not a single dinosaur fossil has ever been found.
In early adolescence, Robert found his first close friend, a boy named Terry he’d met outside. Terry was overweight, had also been excluded by other children, and adored microfossils — the little stuff, according to Robert, “that people usually don’t pay any attention to.” The boys volunteered as assistants at a local archaeology museum together. On school breaks, they left Florida for places with climates more given to preservation. They dug fossils in Wyoming and found trail ruts made a century back by Conestoga wagons. In 2004, when Robert was 22, they undertook a dig in South Dakota, unearthing a collection of plant and dinosaur fossils. They were thrilled. While they were at U-Haul getting a trailer on which to load all their new finds, Terry collapsed. Robert called an ambulance and Terry disappeared into the hospital. “A lady came in, and I thought she was going to say, ‘All right, he’s in rough shape, but you can see him in a couple hours.’ She said he was gone.”
There had been a heart condition. “No one had any goddamn idea,” says DePalma, apologizing for his tears. “All of the wonderful stuff that had gone on for both of us was just gone in an instant. It’s like the world disappears for you.”
After long days together looking at the remnants of other creatures, the boys had talked about what to do with their own bodies when they passed. Robert made a mold from the remains of a duck-billed dinosaur, the last dinosaur remnant Terry ever found, mixed his ashes with resin, and encased them in the shape of the fossil.
He found comfort in the field. “There are so many spots where you don’t see anything manmade at all,” he tells me. “And when you’re there, especially without a phone signal, despite the fact that you’re wearing something modern, you look around and you can literally feel like you are in that time period. I would be back there in a heartbeat.”
In July, I met DePalma in Bowman, North Dakota, and joined him on the short drive to Tanis. He had come all the way from Boca Raton for a single day to help a boyish graduate student in need of soil samples for a thesis. He was wearing a leather hat, thick suspenders, and a red backpack that once belonged to Terry. A long scar he didn’t want to talk about ran through his right eyebrow and continued under his eye. Every time someone asks about the scar, he gives a different story. Aliens, he’ll say. A Kraken.
“I’m not used to all this technology,” he said, messing with the dated screen on the rental-car console as mourning doves rose up from the grass. We passed grazing buffalo, horses, cows: “Cows when they’re born are so flippin’ cute.” Prairie chickens hopped along a fence. “I can’t imagine what it would be like to drive a wagon out here,” he said, clearly imagining it. He pointed out distant hills, turned orange by ancient fire. His thoughts flickered in and out of the present.
“Living history,” DePalma told me once, “is definitely one of my passions.” So is “flint napping,” which involves carving flint into stone tools. Robert’s comfort with matter — his facility with rocks and models — does not extend to strings of electrons beneath the threshold of visibility. “I avoided computers for a long time,” he told me, “and most of the first drafts of my papers were on pen and paper.” Still, he loves the lab and is perfectly comfortable at the synchrotron. “I’m like Alan Grant from Jurassic Park, ” he says, “struggling with the seat belt in the helicopter.”
At a lonely white church guarded by a filthy sheepdog, we met up with Erickson, the paleobiologist who first came up with the idea During allegedly stole, and two graduate students. Pete Larson, DePalma’s co-author from the Black Hills Institute, followed in his own truck as we drove onto the ranch.
“Is this a road?” I asked as we pulled onto the grass and our bodies pitched back and forth in the truck.
“This is a good road,” said Erickson.
We followed a fence line. In the soft breeze of a summer day it was easy to follow the story DePalma told: The grass, rippling in the wind, had once been a river, and this pile of gnarled stone the bank on which the river deposited its dead. Rocked by the asteroid, the rivers would have flooded and the fish pitched onto higher ground. Moments after we arrived, DePalma was already inside the place, leaning against the hill with a brush in one hand and a trowel in the other, carving through sediment as he explained dip angles and bedding traces to the students. In the ashen rock, a fish fossil appears rust red, a subtle change in color and texture barely visible to the layperson. “Oh, check it out,” he said. “There’s another fish coming out right there. Right there. And right here, too. That’s the skull of a sturgeon right there. Hey Greg, we got more fish right there.”
“We can’t decide if that’s the start of a footprint or not,” he said, gesturing at what may have been an herbivorous-dinosaur imprint but I would have thought a perfectly ordinary rock, “but we do find footprints in this horizon. It could be. I mean, that’s definitely soft-sediment deformation.”
He worked away some land and unveiled, across the black soil, a white line. “The boundary,” he said, full of asteroid, a weirdly literal transition between the age of dinosaurs and that of mammals. Later he would take a sample out of his pocket, tip dirt onto a microscope, and show me Nerds-like objects he says he gathered from the site. “Melted Yucatán,” he said, “airmailed from Mexico.”
It became clear during our time together that DePalma is beloved in Bowman; a husky-voiced, cigarette-slim worker -stocking shelves at the gas-station convenience store hugged him the moment we walked in (“A fascinating woman,” he called her), and the mohawked, lip-ringed chef who ran one of the only two restaurants open in town on Monday nights did the same. There is a kind of innocent charisma. DePalma is cordial, formal, quaint, an elder millennial clean of our generation’s collective linguistic tics. “Gosh,” he is given to say. “Son of a gun!” He is earnest in a way that engenders a watchful instinct in others.
“He has a history of getting burned by misplacing his trust,” says Liam O’Meallie, a claims adjuster who often accompanies DePalma on digs. “I’ve been protecting him from behind the scenes for a long time.”
When I ask him to tell me the true story of the scar, DePalma hesitates and gives in. It was a car accident. There had been a pileup in another lane. “It was happening so quickly,” he says, “and I crane my head around idiotically, thinking, Oh, I wonder what the hell is going on over there? Let’s have a look! ” A piece of debris flew into his open window, “smacks me right in the face, destroys that side of my glasses.” He drove home using the one eye not covered in blood.
He did not go to the doctor. “I didn’t want to do insurance,” he says, “I didn’t want to go through cops, I didn’t want to do anything. I didn’t want to deal with bureaucracy, screwing around with all the powers that be, the paperwork.” Cops, insurance, a hospital: all the annoying frictions, the tedious conflicts inherent in the time in which Robert actually lives. They struck him as so stressful and time-consuming as to be intolerable. Instead of stitches, then, there would be only Band-Aids and a scar people will ask him about for the rest of his life.
“I wanted,” he says, “to be left alone.”
Academic publishing is not an ideal realm in which to hide from minor conflict. DePalma’s paper had problems During could see as soon as she took the time to look. Some were immediately obvious to those who skimmed it: The isotopes were off. 18C, an isotope that does not exist, is mentioned multiple times. “Even if it’s just a typo,” says Tobin, the Alabama geologist, “it’s a typo that’s consistent throughout in a way that makes you think, Does anyone on this paper know about isotopes? ”
Others were less obvious and more concerning. During wondered where he had done this analysis; it is hard to acquire these tools. What mass spectrometer had he used? Why were the graphs so perfect, so seesaw in their uniformity? The figures seemed inconsistent with real-world results, or at least results produced with a computer. Some data points were listed twice. It looked to her as if the fish had eaten exactly the same amount every year.
Nature did not, in fact, retract During’s preliminary acceptance, and her paper came out that February. Her paper cited DePalma’s work nine times; it was built on his claims about Tanis, centered on specimens he had sent her, and ultimately rested on his integrity, which she would spend the next several years attacking.
During wrote to Scientific Reports with her concerns about DePalma’s paper, and on Christmas Eve 2021 DePalma received a disturbing email from the journal asking, among other things, about the isotope data. He was “gutted, full of anxiety,” his holiday ruined. Scientific Reports wanted to know the provenance of the raw data, but he did not have it because the person who had handled the data, Curtis McKinney, had died in 2017 before During even got to Tanis. McKinney worked at Miami Dade College. The college did not have a mass spectrometer, so McKinney would have had to do the analysis somewhere else. No one, including DePalma, could say where that was.
No one who knew McKinney seemed to remember him working on such a project. From a certain distance, it could look very much like DePalma had simply found a dead man to whom to attribute work never done. Even his defenders would find the lack of a paper trail a problem. After asking Scientific Reports for more information about the data used in the paper and making no progress in a year’s time, During and Ahlberg were done waiting. They took the extraordinary step of making public a technical 25-page response that was also an accusation. “We are compelled to ask,” they wrote, “whether the data may be fabricated, created to fit an already known conclusion.” (“It was way too personal for my taste,” says Smit. “It was not an objective scientific statement.”) The accusation was covered by Science magazine. In a response to Science, DePalma said the graphs were a bit off because he had plotted them, extraordinarily, by hand. It seemed to him that he was being attacked from all sides. He lost weight. He lost sleep. He was reminded, he tells me, of being bullied as a child.
During and Ahlberg formally complained to the University of Manchester, where DePalma had transferred, alleging misconduct. DePalma formally complained to Uppsala University, where During was a graduate student, alleging plagiarism. “Robert DePalma created these ideas and methods, and … over a period of years Melanie During progressively claimed these ideas for herself, culminating now in these false and shameless accusations against Robert DePalma,” wrote Professor Roy Wogelius in an internal statement to the University of Manchester. “I have been a professional scientist for 40 years and I have never encountered such an unwarranted, unhinged, and hypocritical attack on a colleague.”
“I think she treated Robert very badly,” Larson tells me, “and she, in doing so, probably ruined her own career, which is super-sad for me because she’s a bright young lady.”
DePalma was scheduled to give a talk at TEDx Boca Raton, but he postponed. During gave a talk at TEDx Stockholm. “I knew it was my responsibility to blow the whistle,” she told the crowd. “And why is it important to stand up for science? Because science denial is rampant. Being a researcher means more than doing research … it also takes the backbone to call out misconduct when you see it.”
To his university, now compelled to investigate its own student, DePalma provided copies of the materials he says he gave to During in 2017 when she arrived at Tanis. (She denies having received them.) A page of those materials mentioned “work from Curtis,” strong evidence that McKinney had not been summoned to operate a mass spectrometer from beyond the grave, though to this day no one knows where that analysis took place.
Robert DePalma is, in 2024, 43 years old and still a graduate student, despite having been handed what may or may not be the most spectacular paleontological site in the country. He is by many accounts a brilliant geologist. A student of his I spoke to described him as an extraordinary teacher. But he is not academic. He struggles with parts of paleontology that do not belong in a living-history segment. He does not enjoy publishing; he sees forward to the possibility of criticism. “The dread is, Oh God, then there’s going to be jealous people, ” he says. “There’s going to be people kicking you in the tail over it.” DePalma’s master’s thesis is, according to Smit, unreadable.
In our time in the field, DePalma would almost never mention, without prompting, the world of academic paleontology. He talked about how cool it would be to 3-D print a fossil. He talked about making a film, maybe anime, maybe CGI, from the perspective of an animal that died when the asteroid struck. He showed me a delicate, in-process reconstruction of a rattlesnake. Once in grad school, he had gone to enormous lengths to reconstruct a model of a lungfish in its burrow. “Why did you do this?” he recalls a professor asking him. “Are you studying this? Is this for a paper?”
“I told him time and again,” Smit says, “‘You lay your claims by going to a conference, give a talk, give an abstract.’ And apparently he thought, No, no, no. Then I’m giving away the science. And I couldn’t convince him of the contrary. It’s an imaginary problem for him, so he kept silent about it for a long time. He kept silent about the feathers. He kept silent about the discoveries. And now with Melanie, it sort of overtakes him. I mean, you cannot keep silent for eight years and then not publish anything. That’s not what you do in the modern world. That’s why I say he is a Romantic from the 19th century, but not in a modern, highly competitive field of science. And Melanie is quite the opposite.”
Tanis is so low, DePalma says, because it was part of a river that sliced deep into the land. If Tanis were part of a waterway, that waterway would once have continued onward, and one might therefore find other sites along the ghost of the river packed with petrified carcasses, showered in spherules. In 2023, During and Clint Boyd, a senior paleontologist at the North Dakota Geological Survey, parked a truck next to the ranch where Tanis is located and where Robert was then excavating. They would hike on public land to a Tanis-adjacent spot, also on public land, that During had a feeling about. The hike was hard, over grassy knolls and soft buttes that crumbled under their feet, in part because it was so important to stay on the section line, which was public, though that stretch of land was not always easiest to traverse. They kept checking Boyd’s phone to make sure they weren’t wandering onto someone’s property, until the phone was close to dead. Two hours later, they arrived. Melanie could see the three horns of a triceratops skull sticking out of the side of a hill. They felt more certain this was a solid spot, but it was late in the afternoon and they had a two-hour walk back. They decided to return the next day.
When they were almost back to the truck, a sedan approached. A woman rolled down the passenger-side window. “Better not go in Robert’s section,” she said. It was the woman who helped run the ranch, who had forced out Rob Sula long ago.
From the sedan, the woman gestured behind her.
“I don’t know what those guys want,” she said.
Next to Boyd’s truck were now two pickups and two men. One yelled at them for “trespassing,” spewing a string of expletives; the other paced back and forth to his truck. During was worried about what was in the truck. She hid behind Boyd, whose response to all the yelling was to calmly explain, with an interest that bordered on passion, the complicated rules of North Dakota backcountry private-public byways.
The younger guy got closer to Boyd’s face, waved his arms in protest. Boyd said they would leave.
“You can leave,” he said, “and we are going to watch you leave.”
The men watched them leave. Boyd did not think it was a good idea to go back to the spot on that trip. During flew home.
In December, the University of Manchester’s investigation finally concluded that its doctoral student Robert DePalma had not fabricated data and was probably already working on seasonality before During showed up. The university did say he was guilty of “poor research practice” regarding the mysterious origins of the isotope data. In May, Uppsala University concluded that its doctoral student Melanie During was not guilty of plagiarism, unfair representations of authorship, or deviations from good research practice.
“It has been one of the most hurtful and unexpected and discouraging situations of my life,” DePalma says when pressed about his state of mind, years after all of this started. “It has changed my views of optimism about how people will operate. I’m studying the impact that ended the world for so many different species. And it’s almost ironic that that’s the project that destroyed my soul.”
In June of this year, During returned to North Dakota, searching for a new Tanis. She and Boyd hiked against relentless wind, back toward the hill where she had seen the triceratops skull. It was loud and endless; by the end of an hour and a half of hiking, During’s sunglasses were scratched from flying debris. They gathered gallons of dirt from different sections of the hill and hiked back. Later, sifting through the debris back at the lab, watching it crumble through her fingers, During would find no clear evidence of spherules. But the trip had other pleasures. The wind had blown a salamander vertebrate right out of her palm. They’d found a crocodilian jaw with a tooth in it, just lying there on the surface, the spaces where other teeth had been as straight and uniform as a Battleship grid. They’d found three triceratops (“the cows of the Cretaceous,” During calls them), and Boyd had yanked a Chasmosaurus humerus straight out of a hill. They had found two sets of Thescelosaurus vertebrae. Thescelosaurus, long, heavy bipedal dinosaurs, appeared at the very end of the Late Cretaceous, just before the asteroid that would kill them off.
After the asteroid, surviving birds would carry on the genetic legacy of giant reptiles and the continent would enter the strange age of mammals. There would be a superfast 12-foot-tall bear and a muscular, strong-jawed giant pig. There would be a dog-size horse and beavers six feet long. Camels would evolve right here on the plains, and American lions bigger than African ones. The species that took it upon itself to figure it all out, to make a story of the past, would largely overlook these creatures in favor of T. rex and triceratops. The species would construct elaborate rules that govern who may and may not tread on particular pieces of land. It would evolve a complex economy of prestige to accompany the discovery of truths about all that had come before — a shadowy interplay of gatekeeping, mentorship, and recognition. There would be reason for everyone, on every side of a small discovery of a single fact, to feel overlooked and underappreciated, awake to the vastness of time and vulnerable to the smallest slight.
- social studies
- environment
- new york magazine
Most Viewed Stories
- The Creepy Coincidences of the Superyacht Sinking
- Robert F. Kennedy Jr. Allegedly Had Affairs With 37 Women in 2001
- Trump Denies Rambling Post-DNC in Rambling Truth Social Post
- Why Kamala Harris Is Suddenly Surging Among Latinos
- Is Trump Trying to Back Out of Debating Harris?
- How Donald Trump May Dodge Sentencing Before the Election — or Forever
Editor’s Picks

Most Popular
- The Creepy Coincidences of the Superyacht Sinking By Jeff Wise
- Robert F. Kennedy Jr. Allegedly Had Affairs With 37 Women in 2001 By Dan Amira
- Trump Denies Rambling Post-DNC in Rambling Truth Social Post By Margaret Hartmann
- Why Kamala Harris Is Suddenly Surging Among Latinos By Benjamin Hart
- Is Trump Trying to Back Out of Debating Harris? By Nia Prater
- How Donald Trump May Dodge Sentencing Before the Election — or Forever By Elie Honig

What is your email?
This email will be used to sign into all New York sites. By submitting your email, you agree to our Terms and Privacy Policy and to receive email correspondence from us.
Sign In To Continue Reading
Create your free account.
Password must be at least 8 characters and contain:
- Lower case letters (a-z)
- Upper case letters (A-Z)
- Numbers (0-9)
- Special Characters (!@#$%^&*)
As part of your account, you’ll receive occasional updates and offers from New York , which you can opt out of anytime.
- Quantum Physics
- Davisson Germer Experiment
Davisson Germer Experiment - Electron Diffraction
Initial atomic models proposed by scientists could only explain the particle nature of electrons but failed to explain the properties related to their wave nature. C.J. Davisson and L.H. Germer, in the year 1927, carried out an experiment, popularly known as Davisson Germer’s experiment, to explain the wave nature of electrons through electron diffraction. In this article, we will learn about the observations and conclusions of the experiment.
Setup of Davisson Germer Experiment

The experimental arrangement of the Davisson Germer experiment is discussed below:
- An electron gun comprising a tungsten filament F was coated with barium oxide and heated through a low voltage power supply.
- While applying suitable potential difference from a high voltage power supply, the electron gun emits electrons which were again accelerated to a particular velocity.
- In a cylinder perforated with fine holes along its axis, these emitted electrons were made to pass through it, thus producing a fine collimated beam.
- The beam produced from the cylinder is again made to fall on the surface of a nickel crystal. Due to this, the electrons scatter in various directions.
- The beam of electrons produced has a certain amount of intensity which is measured by the electron detector and after it is connected to a sensitive galvanometer (to record the current), it is then moved on a circular scale.
- By moving the detector on the circular scale at different positions that is changing the θ (angle between the incident and the scattered electron beams), the intensity of the scattered electron beam is measured for different values of angle of scattering.
Read More: Wave Nature of Matter
Observations of Davisson Germer experiment:
From this experiment, we can derive the below observations:
- We obtained the variation of the intensity (I) of the scattered electrons by changing the angle of scattering, θ.
- By changing the accelerating potential difference, the accelerated voltage was varied from 44V to 68 V.
- With the intensity (I) of the scattered electron for an accelerating voltage of 54V at a scattering angle θ = 50º, we could see a strong peak in the intensity.
- This peak was the result of constructive interference of the electrons scattered from different layers of the regularly spaced atoms of the crystals.
- With the help of electron diffraction, the wavelength of matter waves was calculated to be 0.165 nm.
Co-relating Davisson Germer Experiment and de Broglie Relation:
According to de Broglie,
\(\begin{array}{l}λ = (\frac{1.227}{\sqrt{54}}) = 0.167 nm\end{array} \)
λ = wavelength associated with electrons
Thus, Davisson Germer experiment confirms the wave nature of electrons and the de Broglie relation.
Watch the video and learn more about Davisson Germer Experiment

To learn more about Davisson Germer experiment, and other related topics like a magnetic field , electric current, etc. download BYJU’S – The Learning App.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
| PHYSICS Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

COMMENTS
The Davisson-Germer experiment proved beyond doubt the wave nature of matter by diffracting electrons through a crystal. In 1929, de Broglie was awarded the Nobel Prize for his matter wave theory and for opening up a whole new field of Quantum Physics. The matter-wave theory was gracefully incorporated by Heisenberg's Uncertainty Principle.
De Broglie's Hypothesis says that Matter consists of both the particle nature as well as wave nature. De Broglie wavelength λ is given as λ = h p, where p represents the particle momentum and can be written as: λ = h m v Where, h is the Planck's constant, m is the mass of the particle, and v is the velocity of the particle.; From the above relation, it can be said that the wavelength of the ...
The wavelength is known as the de Broglie wavelength. For an electron, de Broglie wavelength equation is: λ =. h mv h m v. Here, λ points to the wave of the electron in question. M is the mass of the electron. V is the velocity of the electron. Mv is the momentum that is formed as a result.
👉Previous Video :https://www.youtube.com/watch?v=Q-w2kfXk2es ️📚👉 Watch Full Free Course: https://www.magnetbrains.com ️📚👉 Get Any Class & Subject's ...
For Physics, Chemistry, Biology & Science Handwritten Notes for Class 10th, 11th, 12th, NEET & JEEDownload App: https://play.google.com/store/apps/details?...
Class 12 Physics https://www.youtube.com/@DynamicVidyapeeth/playlists?view=50&sort=dd&shelf_id=2Chapter 1, Electric Charges and Fields https://youtube.com/pl...
Hence we can verify the de Broglie equation if we observe the motion of an electron. This was done in the Davisson and Germer Experiment. Solved Examples For You. The de-Broglie wavelength of an electron (mass 1 × 10 − 30 k g, charge = 1.6 × 10 − 19 C) with a kinetic energy of 200 e V is: (Planck's constant 6.6 × 10 − 34 J):
Download Article (Size: 117K) The De Broglie Hypothesis. De Broglie argued with a great amount of insight that since nature loves symmetry and simplicity in physical phenomena, ordinary "particles" such as electrons, and protons should also exhibit wave characteristics under certain circumstances.
According to the de-Broglie hypothesis, a moving material particle behaves like a wave at times and like a particle at other times. Every moving material particle is connected with a wave. The de-Broglie wave or matter-wave is an unseen wave associated with a moving particle that propagates in the form of wave packets with the group velocity.
Calculate the de Broglie wavelength of an electron of kinetic energy 500 eV. Solution: The kinetic energy of an electron 500 eV means the electron is accelerated by the potential 500 V. So, the de Broglie wavelength associated with the electron, λ = \(\frac{12.27}{\sqrt{500}}\) = 0.55 Å
De Broglie proposed that a moving material particle of total energy E and momentum p has a wave associated with it (analogous to a photon). He suggested a relation between properties of the wave, like frequency and wavelength, with that of a particle, like energy and momentum. p = E c hv c h E c = hv c = h λ. Thus, the frequency and wavelength ...
The De Broglie hypothesis suggests that all matter exhibits wave-like properties. In particular, the momentum of a particle is related to its wavelength where the De Broglie wavelength may be deduced by the following formula; where p is momentum, h is Planck's constant, λ is wavelength, m is mass, and v is velocity. ... 12.2 - Nuclear ...
E= energy. h = Plank's constant. ν = frequency. From (1) and (2), mc 2 = hν ——- (3) Frequency, ν can be expressed in terms of wavelength, λ as, For a general particle, c can be replaced with the velocity of object, v. Hence, equation (3) can be given as, The above equation is known as de Broglie relationship and the wavelength, λ ...
The de Broglie equation is one of the equations that is commonly used to define the wave properties of matter. It basically describes the wave nature of the electron. Electromagnetic radiation exhibits the dual nature of a particle (having a momentum) and wave (expressed in frequency and wavelength).
de-Broglie Hypothesis _ Grade 12 Physics Notes _ NEB Notes - Free download as PDF File (.pdf), Text File (.txt) or read online for free. De Broglie hypothesis states that all matter has an associated wavelength, known as the de Broglie wavelength. The de Broglie wavelength is inversely proportional to momentum and can be calculated using the formula λ = h/mv, where h is Planck's constant, m ...
De Broglie Hypothesis. Louis de Broglie reasoned that if matter functioned with waves in the very same way that light did, the Planck equation might be applicable to matter as well. As per the de Broglie equation, matter can function as waves in the same way as light and radiation do. The equation acknowledges that a beam of electrons can be ...
According to De-Broglie, the waves are associated with _____ An electron is accelerated through a potential of 120 V. Find its de Broglie wavelength. Calculate De Broglie's wavelength of the bullet moving with speed 90m/sec and having a mass of 5 gm. Explain De Broglie's Hypothesis. The momentum of a photon of energy 1 MeV in kg m/s will be _____
In 1924, Louis de Broglie hypothesised that all matter is wave-like. It means that particles (like atoms and electrons) have both a particle and wave nature and that the wave nature of particles can only be observed when they are being followed. The wavelength of a particle is inversely proportional to its momentum.
️📚👉 Watch Full Free Course:- https://www.magnetbrains.com ️📚👉 Get Notes Here: https://www.pabbly.com/out/magnet-brains ️📚👉 Get All Subjects ...
Revision notes on 12.2.8 De Broglie's Hypothesis for the AQA A Level Physics syllabus, written by the Physics experts at Save My Exams. ... 12.2.8 De Broglie's Hypothesis; 12.2.9 Electron Diffraction; ... Dan graduated with a First-class Masters degree in Physics at Durham University, specialising in cell membrane biophysics. ...
The "Alvarez Hypothesis" was published in 1980, shortly after scientists found a layer of iridium locked in rock the world over and surmised that it could only have come from space.
The thermal de Broglie wavelength (λ th) is approximately the average de Broglie wavelength of the gas particles in an ideal gas at the specified temperature. The thermal de Broglie wavelength is given by the expression: λ D = h / √ 2 π m k B T. where, h = Planck constant. m = mass of a gas particle.
Co-relating Davisson Germer Experiment and de Broglie Relation: According to de Broglie, λ = h /p. λ = wavelength associated with electrons. Thus, Davisson Germer experiment confirms the wave nature of electrons and the de Broglie relation.