

Type 1 Diabetes Mellitus Clinical Presentation
- Author: Romesh Khardori, MD, PhD, FACP; Chief Editor: George T Griffing, MD more...
- Sections Type 1 Diabetes Mellitus
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Complications
- Laboratory Studies
- Tests to Differentiate Type 1 from Type 2 Diabetes
- Approach Considerations
- Self-Monitoring of Glucose Levels
- Continuous Glucose Monitoring
- Insulin Therapy
- Management of Hypoglycemia
- Management of Hyperglycemia
- Management of Complications
- Glycemic Control During Serious Medical Illness and Surgery
- Glycemic Control During Pregnancy
- Consultations
- Medication Summary
- Antidiabetics, Insulins
- Antidiabetics, Amylinomimetics
- Hypoglycemia Antidotes
- Monoclonal Antibodies
- Allogeneic Islet Cells
- Questions & Answers
The most common symptoms of type 1 diabetes mellitus (DM) are polyuria, polydipsia, and polyphagia, along with lassitude, nausea, and blurred vision, all of which result from the hyperglycemia itself.
Polyuria is caused by osmotic diuresis secondary to hyperglycemia. Severe nocturnal enuresis secondary to polyuria can be an indication of onset of diabetes in young children. Thirst is a response to the hyperosmolar state and dehydration.
Fatigue and weakness may be caused by muscle wasting from the catabolic state of insulin deficiency, hypovolemia, and hypokalemia. Muscle cramps are caused by electrolyte imbalance. Blurred vision results from the effect of the hyperosmolar state on the lens and vitreous humor. Glucose and its metabolites cause osmotic swelling of the lens, altering its normal focal length.
Symptoms at the time of the first clinical presentation can usually be traced back several days to several weeks. However, beta-cell destruction may have started months, or even years, before the onset of clinical symptoms.
The onset of symptomatic disease may be sudden. It is not unusual for patients with type 1 DM to present with diabetic ketoacidosis (DKA), which may occur de novo or secondary to the stress of illness or surgery. An explosive onset of symptoms in a young lean patient with ketoacidosis always has been considered diagnostic of type 1 DM.
Over time, patients with new-onset type 1 DM will lose weight, despite normal or increased appetite, because of depletion of water and a catabolic state with reduced glycogen, proteins, and triglycerides. Weight loss may not occur if treatment is initiated promptly after the onset of the disease.
Gastrointestinal (GI) symptoms of type 1 DM are as follows:
Nausea, abdominal discomfort or pain, and change in bowel movements may accompany acute DKA
Acute fatty liver may lead to distention of the hepatic capsule, causing right upper quadrant pain
Persistent abdominal pain may indicate another serious abdominal cause of DKA (eg, pancreatitis
Chronic GI symptoms in the later stage of DM are caused by visceral autonomic neuropathy
Neuropathy affects up to 50% of patients with type 1 DM, but symptomatic neuropathy is typically a late development, developing after many years of chronic prolonged hyperglycemia. Peripheral neuropathy presents as numbness and tingling in both hands and feet, in a glove-and-stocking pattern; it is bilateral, symmetric, and ascending.
History in patients with established diabetes
It is important to inquire about the type and duration of the patient’s diabetes and about the care the patient is receiving for diabetes. Determination of the type of diabetes is based on history, therapy, and clinical judgment. The chronic complications of diabetes are related to the length of time the patient has had the disease.
Ask about the type of insulin being used, delivery system (pump vs injections), dose, and frequency. Also ask about oral antidiabetic agents, if any. Of course, a full review of all medications and over-the-counter supplements being taken is crucial in the assessment of patients with type 1 DM.
Patients using a pump or a multiple-injection regimen have a basal insulin (taken through the pump or with the injection of a long-acting insulin analogue) and a premeal rapid-acting insulin, the dose of which may be determined as a function of the carbohydrate count plus the correction (to adjust for how high the premeal glucose level is). In these patients, ask about the following:
Basal rates (eg, units per hour by pump, generally 0.4-1.5 U/h, potentially varying on the basis of time of day); the total daily dose as basal insulin is a helpful value to know
Carbohydrate ratio (ie, units of insulin per grams of carbohydrate, generally 1 unit of rapid-acting insulin per 10-15 g carbohydrate)
Correction dose (ie, how far the blood glucose level is expected to decrease per unit of rapid-acting insulin, often 1 U of insulin per 50-mg/dL decrease, though individuals with insulin resistance may need 1 U per 25-mg/dL decrease)
Some patients may be taking premeal pramlintide (an amylin analogue)
A focused diabetes history should also include the following questions:
Is the patient’s diabetes generally well controlled, with near-normal blood glucose levels? (Patients with poorly controlled blood glucose levels heal more slowly and are at increased risk for infection and other complications)
Does the patient have severe hypoglycemic reactions? (If the patient has episodes of severe hypoglycemia and therefore is at risk for losing consciousness, this possibility must be addressed, especially if the patient drives)
Does the patient have diabetic nephropathy that might alter the use of medications or intravenous (IV) radiographic contrast material?
Does the patient have macrovascular disease, such as coronary artery disease (CAD), which should be considered in the emergency department (ED)?
Does the patient self-monitor his or her blood glucose levels? (Note the frequency and range of values at each time of day; an increasing number of patients monitor with continuous sensors)
When was the patient’s hemoglobin A 1c (HbA 1c ) value (an indicator of long-term glucose control) last measured? What was it?
In assessing glycemic exposure of a patient with established type 1 DM, review of self-monitored blood glucose levels is necessary. Ideally, this done by uploading time- and date-stamped levels from the patient’s meter to assure full understanding of the frequency of testing and the actual levels.
Questions regarding hypoglycemia and hyperglycemia
Hypoglycemia and hyperglycemia should be considered. Ask the following questions as needed:
Has the patient experienced recent polyuria, polydipsia, nocturia, or weight loss?
Has the patient had episodes of unexplained hypoglycemia? If so, when, how often, and how does the patient treat these episodes?
Does the patient have hypoglycemia unawareness (ie, does the patient lack the adrenergic warning signs of hypoglycemia)? (Hypoglycemia unawareness indicates an increased risk of subsequent episodes of hypoglycemia)
Questions regarding microvascular complications
Microvascular complications, such as retinopathy and nephropathy, should be considered as well. Ask the following questions as appropriate:
When was the patient’s last dilated eye examination? What were the results?
Does the patient have known kidney disease?
What were the dates and results of the last measurements of urine protein and serum creatinine levels?
Questions regarding macrovascular complications
Macrovascular complications should be explored. Questions should include the following:
Does the patient have hypertension? What medications are taken?
Does the patient have symptoms of claudication or a history of vascular bypass?
Has the patient had a stroke or transient ischemic attack?
What are the patient’s most recent lipid levels?
Is the patient taking lipid-lowering medication?
Questions regarding neuropathy
Potential neuropathy should be taken into account. Ask whether the patient has a history of neuropathy or symptoms of peripheral neuropathy or whether autonomic neuropathy is present (including erectile dysfunction if the patient is a man).
Other questions
The possibility of foot disease should be addressed. Inquire as to whether the patient has a history of foot ulcers or amputations or whether any foot ulcers are present. (See Diabetic Foot and Diabetic Foot Infections .)
The possibility of infection also should be considered. Be sure to inquire about whether frequent infections are a problem and, if so, at what sites.
In new cases of diabetes, physical examination findings are usually normal. Patients with DKA, however, will have Kussmaul respiration, signs of dehydration, hypotension, and, in some cases, altered mental status.
In established cases, patients should be examined every 3 months for macrovascular and microvascular complications. They should undergo funduscopic examination for retinopathy and monofilament testing for peripheral neuropathy.
Diabetes-focused examination
A diabetes-focused physical examination includes assessment of vital signs, funduscopic examination, limited vascular and neurologic examinations, and foot examination. Other organ systems should be assessed as indicated by the patient’s clinical situation. A comprehensive examination is not necessary at every visit.
Assessment of vital signs
Patients with established diabetes and autonomic neuropathy may have orthostatic hypotension. Orthostatic vital signs may be useful in assessing volume status and in suggesting the presence of an autonomic neuropathy. Measurement of the pulse is important, in that relative tachycardia is a typical finding in autonomic neuropathy, often preceding the development of orthostatic hypotension. If the respiratory rate and pattern suggest Kussmaul respiration, DKA must be considered immediately, and appropriate tests must be ordered.
Funduscopic examination
The funduscopic examination should include a careful view of the retina. Both the optic disc and the macula should be visualized. If hemorrhages or exudates are seen, the patient should be referred to an ophthalmologist as soon as possible. Examiners who are not ophthalmologists tend to underestimate the severity of retinopathy, which cannot be evaluated accurately unless the patients’ pupils are dilated.
Foot examination
The dorsalis pedis and posterior tibialis pulses should be palpated and their presence or absence noted. This is particularly important in patients who have foot infections: poor lower-extremity blood flow can delay healing and increase the risk of amputation.
Documenting lower-extremity sensory neuropathy is useful in patients who present with foot ulcers because decreased sensation limits the patient’s ability to protect the feet and ankles. If peripheral neuropathy is found, the patient should be made aware that foot care (including daily foot examination) is very important for the prevention of foot ulcers and lower-extremity amputation. (See Diabetic Foot and Diabetic Foot Infections .)
Infections cause considerable morbidity and mortality in patients with diabetes. Infection may precipitate metabolic derangements, and conversely, the metabolic derangements of diabetes may facilitate infection. (See Infections in Patients with Diabetes Mellitus .)
Patients with long-standing diabetes tend to have microvascular and macrovascular disease with resultant poor tissue perfusion and increased risk of infection. The ability of the skin to act as a barrier to infection may be compromised when the diminished sensation of diabetic neuropathy results in unnoticed injury.
Diabetes increases susceptibility to various types of infections. The most common sites are the skin and urinary tract. Dermatologic infections that occur with increased frequency in patients with diabetes include staphylococcal follicular skin infections, superficial fungal infections, cellulitis, erysipelas, and oral or genital candidal infections. Both lower urinary tract infections and acute pyelonephritis are seen with greater frequency.
A few infections, such as malignant otitis externa, rhinocerebral mucormycosis, and emphysematous pyelonephritis, occur almost exclusively in patients with diabetes, though they are fairly rare even in this population. Infections such as staphylococcal sepsis occur more frequently and are more often fatal in patients with diabetes than in others. Infections such as pneumococcal pneumonia affect patients with diabetes and other patients with the same frequency and severity. [ 84 ]
A study reported that out of 178 adult patients hospitalized with coronavirus disease 2019 (COVID-19), at least one underlying condition was found in 89.3%, the most common being hypertension (49.7%), obesity (48.3%), chronic lung disease (34.6%), diabetes mellitus (28.3%), and cardiovascular disease (27.8%). [ 85 ]
According to a report by Stokes et al, out of 287,320 US cases of COVID-19 in which the patient’s underlying health status was known, diabetes was the second most common underlying condition (30%), after cardiovascular disease (32%), which in this study included hypertension. [ 86 , 87 ]
The aforementioned study by Barrera et al found the overall prevalence of diabetes in patients with COVID-19 to be 12%, with the prevalence being 18% in severe COVID-19. [ 63 , 64 ]
In patients with type 1 DM who were diagnosed with COVID-19, a study by Ebekozien et al found that high blood glucose (48.5%), elevated temperature (45.5%), dry cough (39.4%), excess fatigue (33.3%), vomiting (33.3%), shortness of breath (30.3), nausea (30.2%), and body aches/headaches (21.2%) were the most prevalent presenting symptoms reported. Moreover, diabetic ketoacidosis was the most prevalent adverse outcome (45.5%) among these patients. [ 88 , 89 ]
The Centers for Disease Control and Prevention (CDC) includes type 2 DM in the list of conditions that increase the likelihood of severe illness in persons with COVID-19, and type 1 DM in the list of conditions that may increase this likelihood. [ 90 ]
Ophthalmologic complications
Diabetes can affect the lens, vitreous, and retina, causing visual symptoms that may prompt the patient to seek emergency care. Visual blurring may develop acutely as the lens changes shape with marked changes in blood glucose concentrations.
This effect, which is caused by osmotic fluxes of water into and out of the lens, usually occurs as hyperglycemia increases, but it also may be seen when high glucose levels are lowered rapidly. In either case, recovery to baseline visual acuity can take up to a month, and some patients are almost completely unable to read small print or do close work during this period.
Patients with diabetes tend to develop senile cataracts at a younger age than persons without diabetes. Rarely, patients with type 1 DM that is very poorly controlled (eg, those with frequent episodes of DKA) can acutely develop a “snowflake” (or “metabolic”) cataract. Named for their snowflake or flocculent appearance, these cataracts can progress rapidly and create total opacification of the lens within a few days.
Whether diabetes increases the risk of glaucoma remains controversial; epidemiologic studies have yielded conflicting results. [ 91 ] Glaucoma in diabetes relates to the neovascularization of the iris (ie, rubeosis iridis diabetica).
Diabetic retinopathy is the principal ophthalmologic complication of DM. (See Diabetic Retinopathy .) Diabetic retinopathy is the leading cause of blindness in the United States in people younger than 60 years and affects the eyes in the following different ways:
Background retinopathy involves retinal small vessel abnormality leading to hard exudates, hemorrhages, and microaneurysms; it does not affect acuity
Proliferative retinopathy involves extensive proliferation of new retinal small blood vessels; a sudden loss of vision can occur because of vitreous hemorrhage from proliferating new vessels or retinal detachment
Maculopathy involves edema and hard exudate or retinal ischemia; it causes a marked reduction of acuity
Whether patients develop diabetic retinopathy depends on the duration of their diabetes and on the level of glycemic control. [ 92 , 93 , 94 ] The following are the 5 stages in the progression of diabetic retinopathy:
Dilation of the retinal venules and formation of retinal capillary microaneurysms
Increased vascular permeability
Vascular occlusion and retinal ischemia
Proliferation of new blood vessels on the surface of the retina
Hemorrhage and contraction of the fibrovascular proliferation and the vitreous
The first 2 stages of diabetic retinopathy are jointly referred to as background or nonproliferative retinopathy. Initially, the retinal venules dilate, then microaneurysms (tiny red dots on the retina that cause no visual impairment) appear. The microaneurysms or retinal capillaries become more permeable, and hard exudates appear, reflecting leakage of plasma.
Rupture of intraretinal capillaries results in hemorrhage. If a superficial capillary ruptures, a flame-shaped hemorrhage appears. Hard exudates are often found in partial or complete rings (circinate pattern), which usually include multiple microaneurysms. These rings usually mark an area of edematous retina.
The patient may not notice a change in visual acuity unless the center of the macula is involved. Macular edema can cause visual loss; therefore, all patients with suspected macular edema must be referred to an ophthalmologist for evaluation and possible laser therapy. Laser therapy is effective in decreasing macular edema and preserving vision but is less effective in restoring lost vision. (See Macular Edema in Diabetes .)
Preproliferative (stage 3) and proliferative diabetic retinopathy (stages 4 and 5) are the next phases in the progression of the disease. Cotton-wool spots can be seen in preproliferative retinopathy. These represent retinal microinfarcts from capillary occlusion and appear as off-white to gray patches with poorly defined margins.
Proliferative retinopathy is characterized by neovascularization, or the development of networks of fragile new vessels that often are seen on the optic disc or along the main vascular arcades. The vessels undergo cycles of proliferation and regression. During proliferation, fibrous adhesions develop between the vessels and the vitreous. Subsequent contraction of the adhesions can result in traction on the retina and retinal detachment. Contraction also tears the new vessels, which hemorrhage into the vitreous.
Diabetic nephropathy
About 20–30% of patients with type 1 DM develop evidence of nephropathy, [ 95 ] and all patients with diabetes should be considered to have the potential for renal impairment unless proven otherwise. Chronically elevated blood pressure contributes to the decline in renal function. The use of contrast media can precipitate acute renal failure in patients with underlying diabetic nephropathy. Although most recover from contrast medium–induced renal failure within 10 days, some have irreversible renal failure. (See Diabetic Nephropathy .)
Diabetic neuropathy
In the peripheral nerves, diabetes causes peripheral neuropathy. (See Diabetic Lumbosacral Plexopathy and Diabetic Neuropathy .) The 4 types of diabetic neuropathy are as follows:
Peripheral distal symmetrical polyneuropathy, predominantly sensory
Autonomic neuropathy
Proximal painful motor neuropathy
Cranial mononeuropathy (ie, cranial nerve III, IV, or VI)
Of these 4 types, distal symmetric sensorimotor polyneuropathy (in a glove-and-stocking distribution) is the most common. [ 96 ] Besides causing pain in its early stages, this type of neuropathy eventually results in the loss of peripheral sensation. The combination of decreased sensation and peripheral arterial insufficiency often leads to foot ulceration and eventual amputation.
Acute-onset mononeuropathies in diabetes include acute cranial mononeuropathies, mononeuropathy multiplex, focal lesions of the brachial or lumbosacral plexus, and radiculopathies. Of the cranial neuropathies, the third cranial nerve (oculomotor) is most commonly affected, followed by the sixth nerve (abducens) and the fourth nerve (trochlear).
Patients can present with diplopia and eye pain. In diabetic third-nerve palsy, the pupil is usually spared, whereas in third-nerve palsy due to intracranial aneurysm or tumor, the pupil is affected in 80-90% of cases.
It is important to consider nondiabetic causes of cranial nerve palsies, including intracranial tumors, aneurysms, and brainstem stroke. [ 97 ] Therefore, evaluation should include nonenhanced and contrast-enhanced compute4d tomography (CT) or, preferably, magnetic resonance imaging (MRI). Neurologic consultation is recommended. Acute cranial-nerve mononeuropathies usually resolve in 2-9 months. Acute thrombosis or ischemia of the blood vessels supplying the structure involved is thought to cause these neuropathies.
Macrovascular complications
People with diabetes experience accelerated atherosclerosis, affecting the small arteries of the heart, brain, lower extremity, and kidney. Coronary atherosclerosis often occurs at a younger age and is more severe and extensive than in those without diabetes, increasing the risk of ischemic heart disease. Atherosclerosis of the internal carotid and vertebrobasilar arteries and their branches predisposes to cerebral ischemia.
Severe atherosclerosis of the iliofemoral and smaller arteries of the lower legs predisposes to gangrene. Ischemia of a single toe or ischemic areas on the heel are characteristic of diabetic peripheral vascular disease; these result from the involvement of much smaller and more peripheral arteries.
Atherosclerosis of the main renal arteries and their intrarenal branches causes chronic nephron ischemia, which is a significant component of multiple renal lesions in diabetes. However, not all people with type 1 DM are at risk for nephropathy, because there are some polymorphisms in the various factors involved in its pathogenesis, which can modulate the course of this disease from one person to the other.
Risk factors for macrovascular disease
Macrovascular disease is the leading cause of death in patients with diabetes, causing 65-75% of deaths in this group, compared with approximately 35% of deaths in people without diabetes. Diabetes by itself increases the risk of myocardial infarction (MI) 2-fold in men and 4-fold in women, and many patients have other risk factors for MI as well.
The HbA1c value per se, rather than self-reported diabetes status or other established risk factors, robustly predicts MI odds. Each 1% increment in HbA1c independently predicts 19% higher odds for MI. [ 98 ] The risk of stroke in people with diabetes is double that of nondiabetic people, and the risk of peripheral vascular disease is 4 times that of people without diabetes.
Patients with diabetes may have an increased incidence of silent ischemia. [ 99 ] Diastolic dysfunction is common in patients with diabetes and should be considered in patients who have symptoms of congestive heart failure and a normal ejection fraction.
Aathira R, Jain V. Advances in management of type 1 diabetes mellitus. World J Diabetes . 2014 Oct 15. 5 (5):689-96. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Diagnosis and classification of diabetes mellitus. Diabetes Care . 2010 Jan. 33 Suppl 1:S62-9. [QxMD MEDLINE Link] . [Full Text] .
International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care . 2009 Jul. 32(7):1327-34. [QxMD MEDLINE Link] . [Full Text] .
Vehik K, Beam CA, Mahon JL, et al. Development of Autoantibodies in the TrialNet Natural History Study. Diabetes Care . 2011 Sep. 34(9):1897-1901. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care . 2011 Jan. 34 Suppl 1:S11-61. [QxMD MEDLINE Link] . [Full Text] .
Nainggolan L. Continuous Glucose Monitoring: Navigator Beats Rival Devices. Medscape Medical News. January 14, 2013. Available at https://www.medscape.com/viewarticle/777607 . Accessed: January 24, 2013.
Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A Comparative Effectiveness Analysis of Three Continuous Glucose Monitors. Diabetes Care . 2013 Jan 3. [QxMD MEDLINE Link] .
Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One . 2010 Jul 9. 5(7):e11501. [QxMD MEDLINE Link] . [Full Text] .
Pilia S, Casini MR, Cambuli VM, et al. Prevalence of Type 1 diabetes autoantibodies (GAD and IA2) in Sardinian children and adolescents with autoimmune thyroiditis. Diabet Med . 2011 Aug. 28(8):896-9. [QxMD MEDLINE Link] .
Philippe MF, Benabadji S, Barbot-Trystram L, et al. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas . 2011 Apr. 40(3):359-63. [QxMD MEDLINE Link] .
Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep . 2011 Dec. 11(6):533-42. [QxMD MEDLINE Link] . [Full Text] .
Barchetta I, Riccieri V, Vasile M, et al. High prevalence of capillary abnormalities in patients with diabetes and association with retinopathy. Diabet Med . 2011 Sep. 28(9):1039-44. [QxMD MEDLINE Link] .
Young KA, Snell-Bergeon JK, Naik RG, Hokanson JE, Tarullo D, Gottlieb PA, et al. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care . 2011 Feb. 34(2):454-8. [QxMD MEDLINE Link] . [Full Text] .
Joergensen C, Hovind P, Schmedes A, Parving HH, Rossing P. Vitamin d levels, microvascular complications, and mortality in type 1 diabetes. Diabetes Care . 2011 May. 34(5):1081-5. [QxMD MEDLINE Link] .
Zhang D, Efendic S, Brismar K, Gu HF. Effects of MCF2L2, ADIPOQ and SOX2 genetic polymorphisms on the development of nephropathy in type 1 Diabetes Mellitus. BMC Med Genet . 2010 Jul 28. 11:116. [QxMD MEDLINE Link] . [Full Text] .
Busko M. Phenomenon of 'double diabetes' common among blacks. Medscape Medical News . April 25, 2013. [Full Text] .
Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the Estimated Glucose Disposal Rate (eGDR) as a Measure of Insulin Resistance in an Urban Multiethnic Population With Type 1 Diabetes. Diabetes Care . 2013 Apr 17. [QxMD MEDLINE Link] .
Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature . 1994 Sep 8. 371(6493):130-6. [QxMD MEDLINE Link] .
Steck AK, Barriga KJ, Emery LM, Fiallo-Scharer RV, Gottlieb PA, Rewers MJ. Secondary attack rate of type 1 diabetes in Colorado families. Diabetes Care . 2005 Feb. 28(2):296-300. [QxMD MEDLINE Link] .
Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, Orban T. Concordance for islet autoimmunity among monozygotic twins. N Engl J Med . 2008 Dec 25. 359(26):2849-50. [QxMD MEDLINE Link] .
Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev . 2010 Mar. 9(5):A355-65. [QxMD MEDLINE Link] .
Diabetes Epidemiology Research International Group. Geographic patterns of childhood insulin-dependent diabetes mellitus. Diabetes Epidemiology Research International Group. Diabetes . 1988 Aug. 37(8):1113-9. [QxMD MEDLINE Link] .
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes . 2008 Apr. 57(4):1084-92. [QxMD MEDLINE Link] .
Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature . 1987 Oct 15-21. 329(6140):599-604. [QxMD MEDLINE Link] .
Corper AL, Stratmann T, Apostolopoulos V, Scott CA, Garcia KC, Kang AS, et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science . 2000 Apr 21. 288(5465):505-11. [QxMD MEDLINE Link] .
Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes . 2008 Apr. 57(4):1084-92. [QxMD MEDLINE Link] . [Full Text] .
Noble JA, Johnson J, Lane JA, Valdes AM. Race-specific type 1 diabetes risk of HLA-DR7 haplotypes. Tissue Antigens . 2011 Nov. 78(5):348-51. [QxMD MEDLINE Link] . [Full Text] .
Rotwein P, Yokoyama S, Didier DK, Chirgwin JM. Genetic analysis of the hypervariable region flanking the human insulin gene. Am J Hum Genet . 1986 Sep. 39(3):291-9. [QxMD MEDLINE Link] . [Full Text] .
Pugliese A, Zeller M, Fernandez A Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet . 1997 Mar. 15(3):293-7. [QxMD MEDLINE Link] .
Polychronakos C, Li Q. Understanding type 1 diabetes through genetics: advances and prospects. Nat Rev Genet . 2011 Oct 18. 12(11):781-92. [QxMD MEDLINE Link] .
Concannon P, Chen WM, Julier C, Morahan G, Akolkar B, Erlich HA, et al. Genome-wide scan for linkage to type 1 diabetes in 2,496 multiplex families from the Type 1 Diabetes Genetics Consortium. Diabetes . 2009 Apr. 58(4):1018-22. [QxMD MEDLINE Link] . [Full Text] .
Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ . 2011 Feb 3. 342:d35. [QxMD MEDLINE Link] . [Full Text] .
Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes . 2000 Oct. 49(10):1657-65. [QxMD MEDLINE Link] .
Lempainen J, Tauriainen S, Vaarala O, Mäkelä M, Honkanen H, Marttila J, et al. Interaction of enterovirus infection and cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab Res Rev . 2012 Feb. 28(2):177-85. [QxMD MEDLINE Link] .
Cardwell CR, Stene LC, Joner G, Bulsara MK, Cinek O, Rosenbauer J, et al. Maternal age at birth and childhood type 1 diabetes: a pooled analysis of 30 observational studies. Diabetes . 2010 Feb. 59(2):486-94. [QxMD MEDLINE Link] . [Full Text] .
Henry EB, Patterson CC, Cardwell CR. A meta-analysis of the association between pre-eclampsia and childhood-onset Type 1 diabetes mellitus. Diabet Med . 2011 Aug. 28(8):900-5. [QxMD MEDLINE Link] .
Simpson M, Brady H, Yin X, et al. No association of vitamin D intake or 25-hydroxyvitamin D levels in childhood with risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia . 2011 Nov. 54(11):2779-88. [QxMD MEDLINE Link] .
Melville N. Early Upper-Respiratory Infections Linked to Type 1 Diabetes. Medscape Medical News. Available at https://www.medscape.com/viewarticle/807205 . Accessed: July 8, 2013.
Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory Infections in Early Life and the Development of Islet Autoimmunity in Children at Increased Type 1 Diabetes Risk: Evidence From the BABYDIET Study. JAMA Pediatr . 2013 Jul 1. [QxMD MEDLINE Link] .
Tucker ME. New Global Registry Investigates COVID-19 and New-Onset Diabetes. Medscape Medical News . 2020 Jun 13. [Full Text] .
Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol . 2022 Mar 21. [Full Text] .
Tucker ME. 'Profound Implications': COVID Ups Diabetes Risk 40% a Year Later. Medscape Medical News . 2022 Mar 23. [Full Text] .
Tang X, Uhl S, Zhang T, et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab . 2021 May 19. [QxMD MEDLINE Link] . [Full Text] .
Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab . 2021 May 18. [QxMD MEDLINE Link] . [Full Text] .
Barrett CE, Koyama AK, Alvarez P, et al. Risk for Newly Diagnosed Diabetes >30 Days After SARS-CoV-2 Infection Among Persons Aged MMWR Morb Mortal Wkly Rep</i>. 2022 Jan 7. 71: [Full Text] .
Tucker ME. COVID-19 Associated With Increased Diabetes Risk in Youth. Medscape Medical News . 2022 Jan 10. [Full Text] .
Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 Infection With New-Onset Type 1 Diabetes Among Pediatric Patients From 2020 to 2021. JAMA Netw Open . 2022 Sep 1. 5 (9):e2233014. [QxMD MEDLINE Link] . [Full Text] .
Cromer SJ, Colling C, Schatoff D, et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: Associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complications . 2022 Feb 4. 108145. [QxMD MEDLINE Link] . [Full Text] .
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Available at https://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf . Accessed: January 28, 2011.
Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002-2012. N Engl J Med . 2017 Apr 13. 376 (15):1419-29. [QxMD MEDLINE Link] .
Imkampe AK, Gulliford MC. Trends in Type 1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991-2008. Diabet Med . 2011 Jul. 28(7):811-4. [QxMD MEDLINE Link] .
Tucker ME. IDF Atlas: 1 in 10 Adults Worldwide Now Has Diabetes. Medscape Medical News . 2021 Dec 7. [Full Text] .
Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol . 2017 Nov 30. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Needle in a Haystack: Type 1 Diabetes Arises Equally in Adulthood. Medscape . 2017 Dec 4. [Full Text] .
Harjutsalo V, Maric C, Forsblom C, et al. Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia . 2011 Aug. 54(8):1992-1999. [QxMD MEDLINE Link] .
Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, et al. Association Between Coronary Vascular Dysfunction and Cardiac Mortality in Patients with and without Diabetes Mellitus. Circulation . 2012 Aug 23. [QxMD MEDLINE Link] .
Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. J Diabetes Sci Technol . 2020. [Full Text] .
Tucker ME. Pay Attention to In-Hospital Glucose to Save Lives in COVID-19. Medscape Medical News . 2020 Apr 20. [Full Text] .
Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Newly Published Articles Inform on COVID-19 Risk by Diabetes Type. Medscape Medical News . 2020 Aug 17. [Full Text] .
Wargny M, Potier L, Gourdy P, et al. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia . 2021 Feb 17. [QxMD MEDLINE Link] . [Full Text] .
Davenport L. 1 in 5 Diabetes Patients Hospitalized With COVID-19 Die in 28 Days. Medscape Medical News . 2021 Feb 18. [Full Text] .
Zoler ML. Cleaner data confirm severe COVID-19 link to diabetes, hypertension. MDedge Cardiology News . 2020 Jul 27. [Full Text] .
Barrera FJ, Shekhar S, Wurth R, et al. Prevalence of Diabetes and Hypertension and their Associated Risks for Poor Outcomes in Covid-19 Patients. J Endocr Soc . 2020 Jul 21. [Full Text] .
Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol . 2020 Aug 13. [QxMD MEDLINE Link] . [Full Text] .
Schlesinger S, Neuenschwander M, Lang A, et al. Risk phenotypes of diabetes and association with COVID-19 severity and death: a living systematic review and meta-analysis. Diabetologia . 2021 Apr 28. [QxMD MEDLINE Link] . [Full Text] .
Busko M. Older, Sicker Diabetes Patients Have Worse COVID-19 Prognosis. Medscape Medical News . 2021 Apr 28. [Full Text] .
Vangoitsenhoven R, Martens PJ, van Nes F, et al. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients With Type 1 Diabetes. Diabetes Care . 2020 Oct. 43 (10):e118-9. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Emerging Data on Type 1 Diabetes and COVID-19 Reassuring. Medscape Medical News . 2020 Oct 9. [Full Text] .
Carrasco-Sanchez FJ, Lopez-Carmona MD, Martinez-Marcos FJ, et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med . 2021 Dec. 53 (1):103-16. [QxMD MEDLINE Link] .
Tucker ME. Blood Glucose on Admission Predicts COVID-19 Severity in All. Medscape Medical News . 2020 Nov 30. [Full Text] .
Klonoff DC, Messler JC, Umpierrez GE, et al. Association Between Achieving Inpatient Glycemic Control and Clinical Outcomes in Hospitalized Patients With COVID-19: A Multicenter, Retrospective Hospital-Based Analysis. Diabetes Care . 2020 Dec 15. [QxMD MEDLINE Link] . [Full Text] .
Harding A. Glycemia in Early COVID-19 Hospitalization Linked to Mortality. Reuters Health Information . 2020 Dec 21. [Full Text] .
Tucker ME. Small-fiber neuropathy common at 40 years of type 1 diabetes. Medscape Medical News . September 18, 2013. [Full Text] .
Sveen KA, Karimé B, Jørum E, Mellgren SI, Fagerland MW, Monnier VM, et al. Small- and Large-Fiber Neuropathy After 40 Years of Type 1 Diabetes: Associations with glycemic control and advanced protein glycation: The Oslo Study. Diabetes Care . 2013 Sep 11. [QxMD MEDLINE Link] .
Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA . 2005 Oct 12. 294(14):1782-7. [QxMD MEDLINE Link] .
Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med . 2005 Dec 22. 353(25):2643-53. [QxMD MEDLINE Link] . [Full Text] .
DCCT/EDIC Research Group, de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med . 2011 Dec 22. 365(25):2366-76. [QxMD MEDLINE Link] . [Full Text] .
Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ . 2011 Sep 8. 343:d5364. [QxMD MEDLINE Link] . [Full Text] .
Purnell JQ, Hokanson JE, Cleary PA, Nathan DM, Lachin JM, Zinman B, et al. The Effect of Excess Weight Gain with Intensive Diabetes Treatment on Cardiovascular Disease Risk Factors and Atherosclerosis in Type 1 Diabetes: Results from the Diabetes Control and Complications Trial / Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) Study. Circulation . 2012 Dec 4. [QxMD MEDLINE Link] .
Zheng F, Yan L, Yang Z, Zhong B, Xie W. HbA 1c , diabetes and cognitive decline: the English Longitudinal Study of Ageing. Diabetologia . 2018 Jan 25. [QxMD MEDLINE Link] . [Full Text] .
Melville NA. HbA1c Levels in Diabetes Linked to Cognitive Decline. Medscape Medical News . 2018 Jan 30. [Full Text] .
Tucker ME. Type 1 Diabetes Raises COVID-19 Risk in Kids if A1c Is High. Medscape Medical News . 2021 Mar 22. [Full Text] .
Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med . 1999 Dec 16. 341(25):1906-12. [QxMD MEDLINE Link] .
Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 States, March 1–30, 2020. MMWR . 2020 Apr 8. [Full Text] .
Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep . 2020 Jun 15. [Full Text] .
Franki R. Comorbidities Increase COVID-19 Deaths by Factor of 12. Medscape Medical News . 2020 Jun 17. [Full Text] .
Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 Diabetes and COVID-19: Preliminary Findings From a Multicenter Surveillance Study in the U.S. Diabetes Care . 2020 Jun 5. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. Half of Those With Type 1 Diabetes and COVID-19 Manage at Home. Medscape Medical News . 2020 Jun 11. [Full Text] .
Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): People of Any Age with Underlying Medical Conditions. CDC. Available at https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html . Updated June 25, 2020; Accessed: June 27, 2020.
Wong VH, Bui BV, Vingrys AJ. Clinical and experimental links between diabetes and glaucoma. Clin Exp Optom . 2011 Jan. 94(1):4-23. [QxMD MEDLINE Link] .
Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ . 2006 Jul 18. 175(2):165-70. [QxMD MEDLINE Link] . [Full Text] .
Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr . 2005 Feb. 135(2):323-5. [QxMD MEDLINE Link] .
Hammes HP, Kerner W, Hofer S, et al. Diabetic retinopathy in type 1 diabetes-a contemporary analysis of 8,784 patients. Diabetologia . 2011 Aug. 54(8):1977-1984. [QxMD MEDLINE Link] .
Julius MC, Schatz DA, Silverstein JH. The prevention of type I diabetes mellitus. Pediatr Ann . 1999 Sep. 28(9):585-8. [QxMD MEDLINE Link] .
Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am . 2004 Jul. 88(4):947-99, xi. [QxMD MEDLINE Link] .
Chou KL, Galetta SL, Liu GT, Volpe NJ, Bennett JL, Asbury AK, et al. Acute ocular motor mononeuropathies: prospective study of the roles of neuroimaging and clinical assessment. J Neurol Sci . 2004 Apr 15. 219(1-2):35-9. [QxMD MEDLINE Link] .
Gerstein HC, Islam S, Anand S, et al. Dysglycaemia and the risk of acute myocardial infarction in multiple ethnic groups: an analysis of 15,780 patients from the INTERHEART study. Diabetologia . 2010 Dec. 53(12):2509-17. [QxMD MEDLINE Link] .
Falcone C, Nespoli L, Geroldi D, Gazzaruso C, Buzzi MP, Auguadro C, et al. Silent myocardial ischemia in diabetic and nondiabetic patients with coronary artery disease. Int J Cardiol . 2003 Aug. 90(2-3):219-27. [QxMD MEDLINE Link] .
[Guideline] Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract . 2011 Mar-Apr. 17 Suppl 2:1-53. [QxMD MEDLINE Link] .
[Guideline] Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes . 2009 Sep. 10 Suppl 12:33-42. [QxMD MEDLINE Link] .
Hemoglobin A1c and Mean Glucose in Patients With Type 1 Diabetes: Analysis of data from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial. Diabetes Care . 2011 Mar. 34(3):540-4. [QxMD MEDLINE Link] . [Full Text] .
Mianowska B, Fendler W, Szadkowska A, Baranowska A, Grzelak-Agaciak E, Sadon J, et al. HbA(1c) levels in schoolchildren with type 1 diabetes are seasonally variable and dependent on weather conditions. Diabetologia . 2011 Apr. 54(4):749-56. [QxMD MEDLINE Link] . [Full Text] .
Suzuki S, Koga M, Amamiya S, et al. Glycated albumin but not HbA(1c) reflects glycaemic control in patients with neonatal diabetes mellitus. Diabetologia . 2011 Sep. 54(9):2247-53. [QxMD MEDLINE Link] .
Brooks M. Hemoglobin A1c misses many cases of diabetes. Medscape . 2019 Mar 28. [Full Text] .
McDonald TJ, Colclough K, Brown R, et al. Islet autoantibodies can discriminate maturity-onset diabetes of the young (MODY) from Type 1 diabetes. Diabet Med . 2011 Sep. 28(9):1028-33. [QxMD MEDLINE Link] .
[Guideline] Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). ADA. Available at https://professional.diabetes.org/sites/professional.diabetes.org/files/media/draft_easdada_t1dm_adults_consensusreport_0.pdf . 2021; Accessed: July 19, 2021.
[Guideline] Tucker ME. ADA/EASD draft guidance aims to bring adults with type 1 diabetes out of shadows. MDedge . 2021 Jul 14. [Full Text] .
[Guideline] Tucker ME. 'Push the Bar Higher': New Statement on Type 1 Diabetes in Adults. Medscape Medical News . 2021 Oct 4. [Full Text] .
[Guideline] Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia . 2021 Sep 30. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care . 2014 Jun 16. [QxMD MEDLINE Link] .
Tucker M. First-Ever ADA Guidance Specifically for Type 1 Diabetes. Medscape Medical news. Available at https://www.medscape.com/viewarticle/826854 . Accessed: June 20, 2014.
Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual ß-cell function. Diabetes . 2011 May. 60(5):1599-607. [QxMD MEDLINE Link] .
Lantidra (donislecel) [package insert]. Chicago, IL: CellTrans Inc. June 2023. Available at [Full Text] .
US Food and Drug Administration. FDA Approves First Cellular Therapy to Treat Patients with Type 1 Diabetes. FDA. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-cellular-therapy-treat-patients-type-1-diabetes . June 28, 2023; Accessed: July 3, 2023.
[Guideline] American Diabetes Association. Standards of Medical Care in Diabetes-2018 Abridged for Primary Care Providers. Clin Diabetes . 2018 Jan. 36 (1):14-37. [QxMD MEDLINE Link] . [Full Text] .
The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med . 1993 Sep 30. 329(14):977-86. [QxMD MEDLINE Link] .
Genuth S. Insights from the diabetes control and complications trial/epidemiology of diabetes interventions and complications study on the use of intensive glycemic treatment to reduce the risk of complications of type 1 diabetes. Endocr Pract . 2006 Jan-Feb. 12 Suppl 1:34-41. [QxMD MEDLINE Link] .
Lind M, Bounias I, Olsson M, et al. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet . 2011 Jul 9. 378(9786):140-6. [QxMD MEDLINE Link] .
Tomlin A, Dovey S, Tilyard M. Health outcomes for diabetes patients returning for three annual general practice checks. N Z Med J . 2007 Apr 13. 120(1252):U2493. [QxMD MEDLINE Link] .
Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia . 2011 Feb. 54(2):245-55. [QxMD MEDLINE Link] .
Asvold BO, Sand T, Hestad K, Bjørgaas MR. Cognitive function in type 1 diabetic adults with early exposure to severe hypoglycemia: a 16-year follow-up study. Diabetes Care . 2010 Sep. 33(9):1945-7. [QxMD MEDLINE Link] . [Full Text] .
Sherwood JS, Russell SJ, Putman MS. New and Emerging Technologies in Type 1 Diabetes. Endocrinol Metab Clin North Am . 2020 Dec. 49 (4):667-78. [QxMD MEDLINE Link] . [Full Text] .
Garg SK, Voelmle MK, Beatson CR, et al. Use of Continuous Glucose Monitoring in Subjects With Type 1 Diabetes on Multiple Daily Injections Versus Continuous Subcutaneous Insulin Infusion Therapy: A prospective 6-month study. Diabetes Care . 2011 Mar. 34(3):574-9. [QxMD MEDLINE Link] . [Full Text] .
Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care . 2011 Apr. 34(4):795-800. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Klonoff DC, Buckingham B, Christiansen JS, et al. Continuous glucose monitoring: an endocrine society clinical practice guideline. J Clin Endocrinol Metab . 2011 Oct. 96(10):2968-79. [QxMD MEDLINE Link] .
[Guideline] Tucker ME. ADA 2018 Standards Address Diabetes Drugs With CV Benefit. Medscape . 2017 Dec 8. [Full Text] .
Medtronic, Inc. Medtronic gains approval of first artificial pancreas device system with threshold suspend automation [press release]. September 27, 2013. Available at https://newsroom.medtronic.com/phoenix.zhtml?c=251324&p=irol-newsArticle&ID=1859361&highlight . Accessed: October 7, 2013.
Tucker ME. FDA OKs insulin pump with low-glucose suspend feature. Medscape Medical News . September 27, 2013. [Full Text] .
Tucker ME. FDA Okays Use of Dexcom G5 CGM for Insulin Dosing. Medscape Medical News . 2016 Dec 20. [Full Text] .
Tucker ME. FDA Approves New 'Smart' Continuous Glucose Monitor for Diabetes. Medscape Medical News . 2018 Mar 13. [Full Text] .
Tucker ME. FDA Approves First Implantable Continuous Glucose Monitor. Medscape Medical News . 2018 Jun 21. [Full Text] .
FDA approves first continuous glucose monitoring system with a fully implantable glucose sensor and compatible mobile app for adults with diabetes. FDA. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611454.htm . Jun 21, 2018; Accessed: Jun 25, 2018.
Nelson R, Tucker ME. FDA Approves FreeStyle Libre System for Patients. Medscape Medical News . 2017 Sep 27. [Full Text] .
What is the pancreas? What is an artificial pancreas device system?. US Food and Drug Administration. Available at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ArtificialPancreas/ucm259548.htm . May 16, 2016; Accessed: July 6, 2016.
Tucker ME. Coming Soon: 'Artificial Pancreas' Options for Diabetes. Medscape Medical News . June 20, 2016. [Full Text] .
Boggs W. Round-the-Clock Closed-Loop Glucose Control Leads to Better Outcomes. Medscape . May 13, 2016. [Full Text] .
Renard E, Farret A, Kropff J, et al. Day-and-Night Closed-Loop Glucose Control in Patients With Type 1 Diabetes Under Free-Living Conditions: Results of a Single-Arm 1-Month Experience Compared With a Previously Reported Feasibility Study of Evening and Night at Home. Diabetes Care . 2016 Jul. 39 (7):1151-60. [QxMD MEDLINE Link] .
US Food and Drug Administration. FDA approves first automated insulin delivery device for type 1 diabetes. FDA. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522974.htm . Sep 28, 2016; Accessed: Sep 30, 2016.
Busko M. FDA Approves Artificial Pancreas for Children With Type 1 Diabetes. Medscape Medical News . 2018 Jun 22. [Full Text] .
US Food and Drug Administration. FDA Approves First-of-its-Kind Automated Insulin Delivery and Monitoring System for Use in Young Pediatric Patients. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-automated-insulin-delivery-and-monitoring-system-use-young-pediatric . August 31, 2020; Accessed: December 1, 2020.
Tucker M. FDA Approves Inhaled Insulin Afrezza for Diabetes. Medscape Medical News. Available at https://www.medscape.com/viewarticle/827539. . Accessed: July 14, 2014.
Afrezza (insulin inhaled) prescribing information [package insert]. Valencia CA, United States: MannKind Corporation. June, 2014. Available at [Full Text] .
US Food and Drug Administration. Mixups between Insulin U-100 and U-500 (April 2008). FDA Patient Safety News. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/psn/transcript.cfm?show=79 . Accessed: January 28, 2012.
de la Pena A, Riddle M, Morrow LA, et al. Pharmacokinetics and pharmacodynamics of high-dose human regular u-500 insulin versus human regular u-100 insulin in healthy obese subjects. Diabetes Care . 2011 Dec. 34(12):2496-501. [QxMD MEDLINE Link] . [Full Text] .
Garg S, Ampudia-Blasco FJ, Pfohl M. Rapid-acting insulin analogues in Basal-bolus regimens in type 1 diabetes mellitus. Endocr Pract . 2010 May-Jun. 16(3):486-505. [QxMD MEDLINE Link] .
Fiasp Product Information [package insert]. 800 Scudders Mill Road, Plainsboro, NJ 08536: Novo Nordisk Inc. September 2017. Available at [Full Text] .
Nainggolan L. FDA Approves New Fast-Acting Insulin, Fiasp, for Diabetes in Adults. Medscape Medical News . 2017 Sep 29. [Full Text] .
Blair HA, Keating GM. Insulin Glargine 300 U/mL: A Review in Diabetes Mellitus. Drugs . 2016 Mar. 76 (3):363-74. [QxMD MEDLINE Link] .
Toujeo. US Food and Drug Administration. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206538s006lbl.pdf . Accessed: 2018 25 Apr.
Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin Degludec in Type 1 Diabetes: A randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care . 2011 Mar. 34(3):661-5. [QxMD MEDLINE Link] . [Full Text] .
Davies MJ, Gross JL, Ono Y, Sasaki T, Bantwal G, Gall MA, et al. Efficacy and safety of insulin degludec given as part of basal-bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes Metab . 2014 Oct. 16 (10):922-30. [QxMD MEDLINE Link] . [Full Text] .
Zinman B, DeVries JH, Bode B, Russell-Jones D, Leiter LA, Moses A, et al. Efficacy and safety of insulin degludec three times a week versus insulin glargine once a day in insulin-naive patients with type 2 diabetes: results of two phase 3, 26 week, randomised, open-label, treat-to-target, non-inferiority trials. Lancet Diabetes Endocrinol . 2013 Oct. 1 (2):123-31. [QxMD MEDLINE Link] .
Nainggolan L. First Launch for Fiasp : 'Ultrafast' Mealtime Insulin Aspart. Medscape . 2017 29 Mar. [Full Text] .
Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab . 2007 Sep. 9(5):648-59. [QxMD MEDLINE Link] .
Suissa S, Azoulay L, Dell'aniello S, et al. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia . 2011 Sep. 54(9):2254-62. [QxMD MEDLINE Link] .
Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia . 2011 Sep. 54(9):2263-71. [QxMD MEDLINE Link] .
Bao J, Gilbertson HR, Gray R, et al. Improving the Estimation of Mealtime Insulin Dose in Adults With Type 1 Diabetes: The Normal Insulin Demand for Dose Adjustment (NIDDA) study. Diabetes Care . 2011 Oct. 34(10):2146-51. [QxMD MEDLINE Link] . [Full Text] .
Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med . 2010 Jul 22. 363(4):311-20. [QxMD MEDLINE Link] .
Busko M. Insulin pump therapy bests injection therapy in large study. Medscape Medical News . August 19, 2013. [Full Text] .
Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia . 2013 Aug 21. [QxMD MEDLINE Link] . [Full Text] .
King BR, Goss PW, Paterson MA, Crock PA, Anderson DG. Changes in Altitude Cause Unintended Insulin Delivery From Insulin Pumps: Mechanisms and implications. Diabetes Care . 2011 Sep. 34(9):1932-3. [QxMD MEDLINE Link] . [Full Text] .
Grunberger G, Abelseth JM, Bailey TS, Bode BW, Handelsman Y, Hellman R. Consensus statement by the american association of clinical endocrinologists/american college of endocrinology insulin pump management task force. Endocr Pract . 2014 May 1. 20(5):463-89. [QxMD MEDLINE Link] .
Babiker A, Datta V. Lipoatrophy with insulin analogues in type I diabetes. Arch Dis Child . 2011 Jan. 96(1):101-2. [QxMD MEDLINE Link] .
Giménez M, Gilabert R, Monteagudo J, Alonso A, Casamitjana R, Paré C, et al. Repeated episodes of hypoglycemia as a potential aggravating factor for preclinical atherosclerosis in subjects with type 1 diabetes. Diabetes Care . 2011 Jan. 34(1):198-203. [QxMD MEDLINE Link] . [Full Text] .
Asvold BO, Sand T, Hestad KA, Bjorgaas MR. Quantitative EEG in type 1 diabetic adults with childhood exposure to severe hypoglycaemia: a 16 year follow-up study. Diabetologia . 2011 Sep. 54(9):2404-8. [QxMD MEDLINE Link] .
Kacerovsky M, Jones J, Schmid AI, et al. Postprandial and fasting hepatic glucose fluxes in long-standing type 1 diabetes. Diabetes . 2011 Jun. 60(6):1752-8. [QxMD MEDLINE Link] . [Full Text] .
Ahmedani MY, Haque MS, Basit A, Fawwad A, Alvi SF. Ramadan Prospective Diabetes Study: the role of drug dosage and timing alteration, active glucose monitoring and patient education. Diabet Med . 2012 Jun. 29(6):709-15. [QxMD MEDLINE Link] .
Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA . 2006 Jun 21. 295(23):2765-79. [QxMD MEDLINE Link] .
Salardi S, Balsamo C, Zucchini S, Maltoni G, Scipione M, Rollo A, et al. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: the influence of HDL cholesterol. Diabetes Care . 2011 Feb. 34(2):424-9. [QxMD MEDLINE Link] . [Full Text] .
de Boer IH, Rue TC, Cleary PA, et al. Long-term Renal Outcomes of Patients With Type 1 Diabetes Mellitus and Microalbuminuria: An Analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Cohort. Arch Intern Med . 2011 Mar 14. 171(5):412-420. [QxMD MEDLINE Link] . [Full Text] .
Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev . 2006 Oct 18. CD006257. [QxMD MEDLINE Link] .
Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation . 2007 Jan 23. 115(3):387-97. [QxMD MEDLINE Link] .
Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis . 2004 Oct 1. 39(7):885-910. [QxMD MEDLINE Link] .
Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA . 2005 Jan 12. 293(2):217-28. [QxMD MEDLINE Link] .
Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care . 2007 Jan. 30(1):162-72. [QxMD MEDLINE Link] .
Margeirsdottir HD, Stensaeth KH, Larsen JR, Brunborg C, Dahl-Jørgensen K. Early signs of atherosclerosis in diabetic children on intensive insulin treatment: a population-based study. Diabetes Care . 2010 Sep. 33(9):2043-8. [QxMD MEDLINE Link] . [Full Text] .
van Dieren S, Nöthlings U, van der Schouw YT, Spijkerman AM, Rutten GE, van der A DL, et al. Non-fasting lipids and risk of cardiovascular disease in patients with diabetes mellitus. Diabetologia . 2011 Jan. 54(1):73-7. [QxMD MEDLINE Link] . [Full Text] .
Lee SH, Kim JH, Kang MJ, et al. Implications of nocturnal hypertension in children and adolescents with type 1 diabetes. Diabetes Care . 2011 Oct. 34(10):2180-5. [QxMD MEDLINE Link] . [Full Text] .
Leiter LA, Lundman P, da Silva PM, et al. Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med . 2011 Nov. 28(11):1343-1351. [QxMD MEDLINE Link] .
Lund SS, Tarnow L, Astrup AS, Hovind P, Jacobsen PK, Alibegovic AC, et al. Effect of adjunct metformin treatment on levels of plasma lipids in patients with type 1 diabetes. Diabetes Obes Metab . 2009 Oct. 11(10):966-77. [QxMD MEDLINE Link] .
Tucker ME. ACC/AHA statin guidelines, with caveats. WebMD. Available at https://www.medscape.com/viewarticle/837138 . Accessed: Dec 24, 2014.
[Guideline] ElSayed NA, Aleppo G, Aroda VR, et al. Introduction and Methodology: Standards of Care in Diabetes-2023. Diabetes Care . 2023 Jan 1. 46 (Supplement_1):S1-S4. [QxMD MEDLINE Link] . [Full Text] .
Tucker ME. ADA Advises New BP, Lipid Targets for People With Diabetes. Medscape Medical News . 2022 Dec 13. [Full Text] .
Marks JB. Perioperative management of diabetes. Am Fam Physician . 2003 Jan 1. 67(1):93-100. [QxMD MEDLINE Link] .
[Guideline] Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med . 2011 Feb 15. 154(4):260-7. [QxMD MEDLINE Link] .
Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med . 2011 Feb 15. 154(4):268-82. [QxMD MEDLINE Link] .
[Guideline] Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care . 2009 Jun. 32(6):1119-31. [QxMD MEDLINE Link] . [Full Text] .
Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control: what is the evidence?. Crit Care Med . 2007 Sep. 35(9 Suppl):S496-502. [QxMD MEDLINE Link] .
Murphy HR, Steel SA, Roland JM, et al. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med . 2011 Sep. 28(9):1060-7. [QxMD MEDLINE Link] .
Diabetes Prevention Trial--Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med . 2002 May 30. 346(22):1685-91.
Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med . 2002 May 30. 346(22):1685-91. [QxMD MEDLINE Link] .
Gale EA, Bingley PJ, Emmett CL, Collier T. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet . 2004 Mar 20. 363(9413):925-31. [QxMD MEDLINE Link] .
Herold KC, Bundy BN, Long SA, and the, Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med . 2019 Aug 15. 381 (7):603-13. [QxMD MEDLINE Link] . [Full Text] .
Sims EK, Bundy BN, Stier K, and the, Type 1 Diabetes TrialNet Study Group. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med . 2021 Mar 3. 13 (583): [QxMD MEDLINE Link] . [Full Text] .
Wherrett DK, Bundy B, Becker DJ, et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet . 2011 Jul 23. 378(9788):319-27. [QxMD MEDLINE Link] .
Orban T, Bundy B, Becker DJ, et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet . 2011 Jul 30. 378(9789):412-9. [QxMD MEDLINE Link] .
[Guideline] Tucker ME. ADA Issues New Guidance on Type 1 Diabetes in Youth. Medscape Medical News . 2018 Aug 10. [Full Text] .
[Guideline] Chiang JL, Maahs DM, Garvey KC, et al. Type 1 Diabetes in Children and Adolescents: A Position Statement by the American Diabetes Association. Diabetes Care . 2018 Sep. 41 (9):2026-44. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2022. Diabetes Care . 2022 Jan 1. 45 (Supplement_1):S1-S2. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] American Diabetes Association Professional Practice Committee. Summary of Revisions: Standards of Care in Diabetes-2024. Diabetes Care . 2024 Jan 1. 47 (Supplement_1):S5-S10. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Jenkins K. ADA Updates Recommendations for Managing Hypertension in Diabetes. Medscape . 2017 Sep 4. [Full Text] .
[Guideline] de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care . 2017 Sep. 40 (9):1273-1284. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes . 2018 Oct. 19 Suppl 27:262-74. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] DiMeglio LA, Acerini CL, Codner E, et al. ISPAD Clinical Practice Consensus Guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes . 2018 Oct. 19 Suppl 27:105-14. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of Diabetes in Older Adults: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab . 2019 May 1. 104 (5):1520-74. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Tucker ME. New Endocrine Society Guidelines Address Diabetes in Older Adults. Medscape Medical News . 2019 Mar 23. [Full Text] .
[Guideline] Tucker ME. More Guidance on 'Vulnerable Subgroup' With Diabetes and COVID-19. Medscape Medical News . 2020 Apr 28. [Full Text] .
[Guideline] Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol . 2020 Apr 23. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons With Diabetes Mellitus. Endocr Pract . 27 (2021):505-37. [Full Text] .
[Guideline] Tucker ME. 'A Better Picture': First AACE Guidelines on Diabetes Technology. Medscape Medical News . 2021 May 31. [Full Text] .
Lei J, Coronel MM, Yolcu ES, et al. FasL microgels induce immune acceptance of islet allografts in nonhuman primates. Sci Adv . 2022 May 13. 8 (19):eabm9881. [QxMD MEDLINE Link] . [Full Text] .
University of Missouri-Columbia. Harvard Scientists Have Developed a Revolutionary New Treatment for Diabetes. SciTechDaily. Available at https://scitechdaily.com/harvard-scientists-have-developed-a-revolutionary-new-treatment-for-diabetes/ . June 12, 2022; Accessed: June 13, 2022.
US Food and Drug Administration. FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management device system. Available at https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices?fbclid=IwAR3TSBssEd4n6b9hR5oe9Bzwmz3su1yQny8bcQeHVi0WFSsvURBh3nPjR-Y . December 13, 2019; Accessed: December 1, 2020.
[Guideline] American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes . 2015 Apr. 33 (2):97-111. [QxMD MEDLINE Link] . [Full Text] .
Brooks M. FDA Clears Blood Test to Help Diagnose Type 1 Diabetes. Medscape Medical News . Aug 21 2014. [Full Text] .
Hsiao-Chuan L, et al. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: A nationwide population-based cohort study. Diabetologia . 2014. [Full Text] .
Leegaard A, Riis A, Kornum JB, et al. Diabetes, Glycemic Control, and Risk of Tuberculosis: A population-based case-control study. Diabetes Care . 2011 Dec. 34(12):2530-5. [QxMD MEDLINE Link] . [Full Text] .
Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med . 2011 Oct 20. 365(16):1509-19. [QxMD MEDLINE Link] .
[Guideline] Peters A, Laffel L. Diabetes Care for Emerging Adults: Recommendations for Transition From Pediatric to Adult Diabetes Care Systems: A position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Socie... Diabetes Care . 2011 Nov. 34(11):2477-85. [QxMD MEDLINE Link] . [Full Text] .
US Food and Drug Administration. Early Communication About Safety of Lantus (Insulin Glargine). Available at https://www.fda.gov/Drugs/DrugSafety/ucm239376.htm . Accessed: May 22, 2012.

Contributor Information and Disclosures
Romesh Khardori, MD, PhD, FACP (Retired) Professor, Division of Endocrinology, Diabetes and Metabolism, Department of Internal Medicine, Eastern Virginia Medical School Romesh Khardori, MD, PhD, FACP is a member of the following medical societies: American Association of Clinical Endocrinology , American College of Physicians , American Diabetes Association , Endocrine Society Disclosure: Nothing to disclose.
George T Griffing, MD Professor Emeritus of Medicine, St Louis University School of Medicine George T Griffing, MD is a member of the following medical societies: American Association for Physician Leadership , American Association for the Advancement of Science , American College of Medical Practice Executives , American College of Physicians , American Diabetes Association , American Federation for Medical Research , American Heart Association , Central Society for Clinical and Translational Research , Endocrine Society , International Society for Clinical Densitometry , Southern Society for Clinical Investigation Disclosure: Nothing to disclose.
Howard A Bessen, MD Professor of Medicine, Department of Emergency Medicine, University of California, Los Angeles, David Geffen School of Medicine; Program Director, Harbor-UCLA Medical Center
Howard A Bessen, MD is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.
Barry E Brenner, MD, PhD, FACEP Professor of Emergency Medicine, Professor of Internal Medicine, Program Director, Emergency Medicine, Case Medical Center, University Hospitals, Case Western Reserve University School of Medicine
Barry E Brenner, MD, PhD, FACEP is a member of the following medical societies: Alpha Omega Alpha , American Academy of Emergency Medicine , American College of Chest Physicians , American College of Emergency Physicians , American College of Physicians , American Heart Association , American Thoracic Society , Arkansas Medical Society , New York Academy of Medicine , New York Academy ofSciences ,and Society for Academic Emergency Medicine
Aneela Naureen Hussain, MD, FAAFM Assistant Professor, Department of Family Medicine, State University of New York Downstate Medical Center; Consulting Staff, Department of Family Medicine, University Hospital of Brooklyn
Aneela Naureen Hussain, MD, FAAFM is a member of the following medical societies: American Academy of Family Physicians , American Medical Association , American Medical Women's Association , Medical Society of the State of New York , and Society of Teachers of Family Medicine
Anne L Peters, MD, CDE Director of Clinical Diabetes Programs, Professor, Department of Medicine, University of Southern California, Keck School of Medicine, Los Angeles, California, Los Angeles County/University of Southern California Medical Center
Anne L Peters, MD, CDE is a member of the following medical societies: American College of Physicians and American Diabetes Association
Disclosure: Amylin Honoraria Speaking and teaching; AstraZeneca Consulting fee Consulting; Lilly Consulting fee Consulting; Takeda Consulting fee Consulting; Bristol Myers Squibb Honoraria Speaking and teaching; NovoNordisk Consulting fee Consulting; Medtronic Minimed Consulting fee Consulting; Dexcom Honoraria Speaking and teaching; Roche Honoraria Speaking and teaching
Don S Schalch, MD Professor Emeritus, Department of Internal Medicine, Division of Endocrinology, University of Wisconsin Hospitals and Clinics
Don S Schalch, MD is a member of the following medical societies: American Diabetes Association , American Federation for Medical Research , Central Society for Clinical Research , and Endocrine Society
Erik D Schraga, MD Staff Physician, Department of Emergency Medicine, Mills-Peninsula Emergency Medical Associates
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
Miriam T Vincent, MD, PhD Professor and Chair, Department of Family Practice, State University of New York Downstate Medical Center
Miriam T Vincent, MD, PhD is a member of the following medical societies: Alpha Omega Alpha , American Academy of Family Physicians , American Association for the Advancement of Science , Medical Society of the State of New York , North American Primary Care Research Group , Sigma Xi , and Society of Teachers of Family Medicine
Disclosure: Joslin Diabetes Group, Harvard Honoraria Speaking and teaching
Scott R Votey, MD Director of Emergency Medicine Residency, Ronald Reagan UCLA Medical Center; Professor of Medicine/Emergency Medicine, University of California, Los Angeles, David Geffen School of Medicine
Scott R Votey, MD is a member of the following medical societies: Society for Academic Emergency Medicine
Frederick H Ziel, MD Associate Professor of Medicine, University of California, Los Angeles, David Geffen School of Medicine; Physician-In-Charge, Endocrinology/Diabetes Center, Director of Medical Education, Kaiser Permanente Woodland Hills; Chair of Endocrinology, Co-Chair of Diabetes Complete Care Program, Southern California Permanente Medical Group
Frederick H Ziel, MD is a member of the following medical societies: American Association of Clinical Endocrinologists , American College of Endocrinology , American College of Physicians , American College of Physicians-American Society of Internal Medicine , American Diabetes Association , American Federation for Medical Research , American Medical Association , American Society for Bone and Mineral Research , California Medical Association , Endocrine Society , andInternational Society for Clinical Densitometry
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Type 1 Diabetes Mellitus
- Pediatric Type 1 Diabetes Mellitus
- Type 2 Diabetes Mellitus
- Pediatric Type 2 Diabetes Mellitus
- Fast Five Quiz: How Much Do You Know About Diabetic Neuropathy?
- Fast Five Quiz: Atrial Fibrillation and Diabetes
- Perioperative Management of the Diabetic Patient
- Diabetes Mellitus Type 1 News & Perspectives
- 'No Excuses': Training for the Olympics with Type 1 Diabetes
- Are Immune Therapies for Type 1 Diabetes Worthwhile?
- Don't Overlook Cardiovascular Risk in Type 1 Diabetes
- Association of High-Density Lipoprotein Parameters and Risk of Heart Failure

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 2002117739-overviewDiseases & Conditions Diseases & Conditions Type 1 Diabetes Mellitus
- 2010trulicity-dulaglutide-999965Drugs Drugs dulaglutide

Faculty and Disclosures
Zachary t bloomgarden, md.
Associate Clinical Professor of Medicine, Mount Sinai Medical School, New York, NY
Disclosure: Zachary T. Bloomgarden, MD, has disclosed that he receives research grant support from Hoechst Marion Roussel, Novartis, and TCPI Inc. He has consulting agreements with Hoechst Marion Roussel, Novartis, Parke-Davis, Bristol-Myers Squibb Company, Novo Nordisk, Pfizer Inc., Eli Lilly and Company, Takeda, and GlaxoSmithKline.

Case Study 1: Patient with Newly Diagnosed Type 1 Diabetes
- Authors: Author: Zachary T. Bloomgarden, MD
- THIS ACTIVITY HAS EXPIRED FOR CREDIT
Target Audience and Goal Statement
This activity is intended for physicians and pharmacists.
This article reviews the physiologic consequences of diabetes mellitus and presents evidence that supports the benefits of aggressive intervention to achieve glycemic control. Real-life clinical scenarios will be presented to illustrate the practical clinical applications of insulin preparations in patients with diabetes.
- Describe the physiologic consequences of diabetes mellitus.
- Outline the importance of maintaining glycemic control in reducing the risk of diabetic complications.
- Detail specific clinical applications of insulin therapy to achieve both basal and meal-related glycemic control.
- Manage a patient's glycemic status by continuously refining the therapeutic approach.
Accreditation Statements
For physicians.
Medical Education Collaborative, a nonprofit education organization, is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians.
Medical Education Collaborative designates this educational activity for a maximum of 1 hour in Category 1 credit towards the AMA Physician's Recognition Award. Each physician should claim only those hours of credit that he/she actually spent in the educational activity.
Contact This Provider
For Pharmacists
Medical Education Collaborative, Inc. has assigned 1 contact hour (0.1 CEUs) of continuing pharmaceutical education credit. ACPE universal program number: 815-999-01-016-H04 . Certificate is defined as a record of participation.
For questions regarding the content of this activity, contact the accredited provider for this CME/CE activity noted above. For technical assistance, contact [email protected]
Instructions for Participation and Credit
- Read the target audience, learning objectives, and author disclosures.
- Study the educational content online or printed out.
- Online, choose the best answer to each test question. To receive a certificate, you must receive a passing score as designated at the top of the test. Medscape encourages you to complete the Activity Evaluation to provide feedback for future programming.
Case 1 History
Results of hospital laboratory studies (Table 1-1) revealed that the patient's initial blood glucose level was 1192 mg/dL and clinical presentation and laboratory findings were consistent with a diagnosis of diabetic ketoacidosis (DKA). The patient reported no family history of diabetes. His father died at age 35 of renal failure.
The patient was treated successfully for DKA and discharged from the hospital 3 days later on an insulin regimen consisting of 30 units of NPH/regular human insulin 70/30 mixture (70/30 mix) before breakfast, 15 units of regular human insulin before dinner, and 20 units of NPH insulin at bedtime.
On discharge, he was instructed to perform blood glucose measurements 4 times a day. The patient was seen as an outpatient 4 days after he is discharged from hospital.
Table 1-1. Hospital Laboratory Studies
2 AM 4 AM 5 AM 9 AM 11 AM 1 PM Plasma Glucose mg/dL 1192 958 718 358 288 222 Sodium mEq/L 154 -- 158 167 -- 161 Potassium mEq/L -- -- 3.3 4.0 -- 3.5 Creatinine (mg/dL) 1.7 1.6 -- -- 0.9 -- pH 7.34 -- -- -- -- -- Urine Acetone -- -- -- 2+ -- --
| This patient presented to the emergency department with acute-onset diabetes with classic symptoms of insulin deficiency compatible with a diagnosis of type 1 diabetes. Approximately 25% of patients that present with DKA have new onset of type 1 diabetes. Antibody testing was not performed, presumably because of the typical type 1 presentation. During his hospital stay, the patient received instructions regarding diet, medication schedule, and home glucose monitoring. The patient was discharged on an insulin regimen designed for ease of administration, consisting of a premixed 70/30 mix before breakfast. The evening insulin regimen included regular insulin before dinner and NPH at bedtime. Because the peak action of NPH is expected approximately 8-10 hours following administration, giving the evening dose of NPH at bedtime rather than before dinner avoids the nocturnal (2-3 AM) hypoglycemia that is often associated with dinnertime NPH administration. |

Nursing Case Study for Type 1 Diabetes
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Michael is a 14-year-old male brought into a small ER by his mother. They were driving a long distance after he competed in a wrestling tournament. He had not felt well on the bus ride with the team so his mother decided he should ride with her. His mother denies a history of chronic illness but did say he had “like a cold but with a stomachache” about 3 months ago.
She also says that he has been very thirsty, and they had to stop several times for him to urinate. She is also worried because he almost missed his wrestling “weight class” parameters because he was significantly lighter this past weekend than he has been in the past. And that is even with him eating more than usual.
What symptoms are most worrisome to the triage nurse?
- He has 2 of the 3 “p’s” – polydipsia (thirst), polyuria (frequent urination), polyphagia (hunger) which are trademarks of diabetes mellitus (DM) and/or diabetic ketoacidosis (DKA). They happen in response to the body lacking insulin and its response is to try to achieve homeostasis with these mechanisms. His weight loss could be indicative of DM as well.
- They describe a recent viral-like illness which may precipitate a diagnosis of DM (it is thought the body has an inappropriate immune response to the illness leading to DM)
In triage, the nurse obtains a point-of-care blood glucose (BG) level and the machine gives no value. Instead, an error message indicating “hi” displays on the machine.
Why did the nurse do this test? What should they do next?
- Clues point to possible DM or DKA. Getting a BG immediately can help guide care. Always follow the facility protocol/procedure on “hi” or “lo” (often spelled this way on glucometers) readings. The protocol might dictate (standing order) stat venous draw and send it to the lab. It may be advised to try again on a different machine with a new sample. Whatever the guidance, a BG level is imperative for this patient.
Michael is AAO x 4. He complains of a “stomachache” and reports he has nausea and experienced vomiting shortly before arrival. His skin is warm and dry, but his face is flushed. When asked about pain, he says he has a headache, and his vision is blurry. The nurse notices a fruity odor on his breath when obtaining vital signs.
BP 90/54 mmHg SpO 2 98% on Room Air
HR 122 bpm and regular
RR 26 bpm at rest
The patient and his mother are placed into an exam room immediately and the triage nurse verbally reports this to the accepting nurse.
How does the nurse interpret these symptoms?
- Michael’s symptoms are consistent with hyperglycemia (link here to cheatsheet?) DKA
What orders does the accepting nurse anticipate?
- Labs, ABGs, urinalysis, IV access (bilateral upper extremities, largest possible in case patient deteriorates). One lab, in particular, can give the provider an idea of the last 2-3 month BG average, the hemoglobin A1C.
The provider orders stat labs, urinalysis and ABGs then examines the patient.
Why stat orders?
- This patient’s condition could deteriorate rapidly, and treatment should begin ASAP. Labs are needed to guide the plan of care. The nurse should watch for changes in the level of consciousness, respiratory changes, his response to potential fluid & electrolyte imbalances. Place on continuous cardiac monitoring as well.
Lab results are as follows:
WBC 15000 cells/mcL
Glucose 420 mg/dl
BUN 21 mg/dl
Creatinine 0.77 mg/dl
Anion gap 12
Glucose positive
Ketones positive
What do these results mean?
- CBC WBC 15000 cells/mcL – an immune response, possibly to viral illness or another issue HgbA1C 9% – indicates the average BG over the past 2-3 months has been about 212mg/dLBMP Glucose 420 mg/dl – hyperglycemia K 5.8 – electrolyte imbalance, can cause cardiac changes and need to monitor closely if IV insulin is started (will need frequent checks of this and BG) BUN 21 mg/dL – fluid imbalance Creatinine 0.77 mg/dL – normal but necessary to check for kidney function Anion gap 12 – indicative of DKAABG – metabolic acidosis Ph 7.25 HCO3 15 PaCo2 35 PaO2 88Urine – indicative of DKA Glucose positive Ketones positive
What medication orders should the nurse anticipate?
- IV fluids, insulin (either IV or SQ). NOTE: only REGULAR INSULIN can be given IV, and if it is, then IV dextrose and potassium chloride should be included in the insulin IV titration protocol/order). SQ insulin may be ordered using a sliding scale. O2 via NC possibly due to potential respiratory concerns (Kussmaul respirations)
The provider tells Michael and his mother that he suspects diabetic ketoacidosis which is not uncommon for new type I diabetics. He plans to transfer Michael to a nearby city via helicopter for a higher level of care. The patient’s mother asks why he has to be transferred.
How does the nurse explain the transfer to the mother and patient?
- DKA requires monitoring in a critical care unit. Because of his age and new-onset DM, a higher level of care is recommended in order to have access to the best resources
The flight team arrives and assesses the patient. The ER completes a report using SBAR format at the bedside. The patient and his mother are given the chance to ask questions.
What are the transport team’s priorities as they move this patient?
- Airway, breathing, and circulation (ABC) status; Mental status; Volume status.
Upon arrival to the higher level of care, Michael is admitted to the ICU overnight. By the morning he is transferred to a pediatric floor for further observation. His mother remains at his bedside. They plan to return to their home after discharge.
How should the pediatric medical unit prepare this family for discharge? What specific teaching should be provided?
- Condition-specific education is vital including DM management with medications, exercise, nutrition, psychosocial concerns, preventative care (i.e. vaccinations), parental/family involvement. A specialized diabetic educator and/or dietician would be ideal. Assessing their education preferences and literacy level is important as well. How to give insulin injections and check BG (glucometer use) are key takeaways (have patient and parent return-demonstrate). Case management may need to get involved for prescription/supplies. An endocrinologist may be consulted so education about his specialist is also important.
References:
View the full outline.
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
View the FULL Transcript
Nursing case studies.

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
A case report: First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35-month-old girl
Affiliations.
- 1 Division of Pediatric Intensive Care Department of Pediatrics Shiraz University of Medical Sciences Shiraz Iran.
- 2 Division of Pediatric Metabolism and Endocrinology Department of Pediatrics Shiraz University of Medical Sciences Shiraz Iran.
- PMID: 34765201
- PMCID: PMC8572339
- DOI: 10.1002/ccr3.4984
Hyperglycemic hyperosmolar syndrome (HHS) is a rare complication of diabetes mellitus among pediatric patients. Since its treatment differs from diabetic ketoacidosis (DKA), hence, pediatricians should be aware of its diagnosis and management.
Keywords: case report; diabetes mellitus; hyperglycemic hyperosmolar syndrome (HHS); pediatric patients; rhabdomyolysis; thrombosis.
© 2021 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
PubMed Disclaimer
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
- Pediatric Diabetic Ketoacidosis With Hyperosmolarity: Clinical Characteristics and Outcomes. Agrawal S, Baird GL, Quintos JB, Reinert SE, Gopalakrishnan G, Boney CM, Topor LS. Agrawal S, et al. Endocr Pract. 2018 Aug;24(8):726-732. doi: 10.4158/EP-2018-0120. Epub 2018 Aug 7. Endocr Pract. 2018. PMID: 30084686
- A Rare Presentation of New-Onset Type 1 Diabetes Mellitus in a Developmentally Delayed Child With an Overlap of Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Parra Villasmil MG, Patel S, Tansey M, Badheka A, Chegondi M. Parra Villasmil MG, et al. Cureus. 2022 Sep 9;14(9):e28983. doi: 10.7759/cureus.28983. eCollection 2022 Sep. Cureus. 2022. PMID: 36237743 Free PMC article.
- Combined diabetic ketoacidosis and hyperosmolar hyperglycemic state in type 1 diabetes mellitus induced by immune checkpoint inhibitors: Underrecognized and underreported emergency in ICIs-DM. Zhang W, Chen J, Bi J, Ding N, Chen X, Wang Z, Jiao Y. Zhang W, et al. Front Endocrinol (Lausanne). 2023 Jan 4;13:1084441. doi: 10.3389/fendo.2022.1084441. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36686495 Free PMC article. Review.
- Severe hypernatremia in soft drink ketoacidosis and hyperglycemic hyperosmolar state at the onset of type 2 diabetes mellitus: a case series of three adolescents. Choo SJ, Lee HG, Kim CJ, Yang EM. Choo SJ, et al. Clin Pediatr Endocrinol. 2022;31(2):81-86. doi: 10.1297/cpe.2021-0075. Epub 2022 Feb 16. Clin Pediatr Endocrinol. 2022. PMID: 35431447 Free PMC article.
- Treatment of Diabetic Ketoacidosis (DKA)/Hyperglycemic Hyperosmolar State (HHS): Novel Advances in the Management of Hyperglycemic Crises (UK Versus USA). Dhatariya KK, Vellanki P. Dhatariya KK, et al. Curr Diab Rep. 2017 May;17(5):33. doi: 10.1007/s11892-017-0857-4. Curr Diab Rep. 2017. PMID: 28364357 Free PMC article. Review.
- Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155‐177. 10.1111/pedi.12701 - DOI - PubMed
- Zeitler P, Haqq A, Rosenbloom A, Glaser N, Drugs and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society . Hyperglycemic hyperosmolar syndrome in children: pathophysiological considerations and suggested guidelines for treatment. J Pediatr. 2011;158(1):9‐14.e142. 10.1016/j.jpeds.2010.09.048 - DOI - PubMed
- Klingensmith GJ, Connor CG, Ruedy KJ, et al. Presentation of youth with type 2 diabetes in the Pediatric Diabetes Consortium. Pediatr Diabetes. 2016;17(4):266‐273. 10.1111/pedi.122814 - DOI - PubMed
- Agrawal S, Baird GL, Quintos JB, et al. Pediatric diabetic ketoacidosis with hyperosmolarity: clinical characteristics and outcomes. Endocr Pract. 2018;24(8):726‐732. 10.4158/EP-2018-0120 - DOI - PubMed
- Rosenbloom AL. Hyperglycemic hyperosmolar state: an emerging pediatric problem. J Pediatr. 2010;156(2):180‐184. 10.1016/j.jpeds.2009.11.057 - DOI - PubMed
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Europe PubMed Central
- PubMed Central

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Arch Clin Cases
- v.8(3); 2021

An atypical presentation of type 1 diabetes
Brandon w. knopp.
1 Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, USA
Parvathi Perumareddi
2 Department of Integrated Medical Science, Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, USA
Type 1 and type 2 diabetes have been described historically as occurring in distinct patient populations; however, atypical demographics are becoming more frequent as the prevalence of diabetes increases, crossing boundaries of ages. Some of these cases can be challenging to diagnose clinically as the patient symptomatology and progression can differ from the standard features of type 1 and 2 diabetes. Our case is an example of a patient whose type 1 diabetes presented atypically with characteristics often associated with type 2 diabetes. Patient presentations such as this are uncommon, with our patient having presented with the “textbook” characteristics of type 2 diabetes. When first diagnosed with diabetes mellitus type 2, the patient was 60 years old, had a BMI around 30 and experienced a gradual onset of symptoms over the course of several months. At the age of 64, the patient tested positive for GAD65 autoantibodies following a year of declining glycemic control and was re-evaluated and classified as a type 1 diabetes patient. Subsequent insulin injections resolved his diabetes-related complications which included polyuria, weakness and weight loss and improved his glycemic control. This case provides an example of an unusual clinical presentation of type 1 diabetes and serves to raise awareness for atypical presentations of diabetes to improve accurate classifications at earlier stages.
Introduction
Diabetes mellitus is a metabolic disease characterized by insulin deficiency and/or insulin resistance. Type 1 diabetes results from the autoimmune destruction of pancreatic β cells and is typically found in younger patients while type 2 diabetes occurs due to insulin resistance and is most often found in middle aged to older adults. Each type has been associated with a distinct patient population, however, there are reports of cases with atypical demographics characterized by a crossover in age, weight and other factors [ 1 , 2 ].
These rare cases are difficult to identify clinically and may require antibody testing to confirm the diagnosis. The incidence of atypical presentations of diabetes mellitus is unknown, however, with the rise in diabetes cases over the past several decades and an expected increase of 54% between 2015 and 2030 [ 3 ], a concomitant increase in atypical cases can be reasonably expected.
Case report
This case describes a 64-year-old Caucasian male who was referred for evaluation and treatment of diabetes mellitus which was first diagnosed at the age of 60. At the age of 60 he presented with minimal clinical signs of diabetes mellitus, including mild fatigue, and was diagnosed with type 2 diabetes due to hyperglycemia and elevated HbA1c. He was treated as a type 2 diabetes patient for four years before being placed on insulin at the age of 64 following a significant deterioration in glycemic control. He had no known risk factors for diabetes mellitus and was screened with self-monitoring of blood glucose (SMBG). His past medical history was significant for elevated thyroid stimulating hormone (TSH), but otherwise no significant abnormalities.

Clinical Progression
On 10/28/2020, the patient presented with a multi-day history of polyuria and weakness with deterioration since his previous visit on 7/3/20. Following a weight loss of 5.4 kg during the previous several days, the patient weighed 84.4 kg with a BMI of 29.1. He rarely checked his blood glucose but measured a value of almost 500 mg/dL at home during this time and a value of close to 400 mg/dL in office. On a physical exam, the patient did not appear weak or ill and reported no changes in vision or neurologic symptoms. He reported consistency in taking metformin and glimepiride, with sertraline being added a few months prior. Because of the significant hyperglycemia and concerns of potential diabetic ketoacidosis, he was injected with 20 units of insulin degludec, given 5 units of insulin lispro with instructions for later use and asked to drink 5 cups of water while in office. ( Note : treatments vary among providers)
At his follow up on 11/5/2020, he improved with no significant incidences of hyperglycemia, though he experienced blood sugar spikes after breakfast. Along with dietary interventions to mitigate post-breakfast spikes, Insulin degludec increased to 23 units daily and 5 units of insulin lispro with meals. Metformin and Glimepiride were discontinued.
During his visit on 3/29/2021, the patient stated his diabetes was not well-controlled and had variable glucose ranges with hypoglycemia typically occurring before dinnertime. He had a temperature of 36.4 o C, weight of 90.7 kg, height of 171.5 cm and BMI of 30.86. The physical exam findings were normal with no foot ulcers or other visible abnormalities. He also had an intact sensory exam. The patient had a recent c-peptide of 0.5 ng/mL on 12/24/2020 and an HbA1c of 7.4% (normal 4.2–5.7%).
During his visit on 5/17/2021, the patient stated his diabetes was well-controlled. He used an insulin pump and continuous glucose monitoring (CGM) sensor between 5/4/2021 and 5/17/2021. Between those dates, his blood glucose was 88% in range with no significant hypoglycemia since beginning insulin pump use. He gained 3.6 kg since 3/29/2021 with no new development of diabetes-related symptoms. Table 1 presents the most important laboratory parameters.
Select laboratory results
| Insulin Growth Factor 1 | 255 ng/mL (high) | Normal: 46-219 ng/mL |
| Fibrinogen | 530 mg/dL (high) | Normal: 276-471 mg/dL |
| Urea nitrogen | 25 mg/dL (high) | Normal: 6-23 mg/dL |
| Creatinine | 1.1 mg/dl | Normal: 0.7–1.3 mg/dl |
Diagnosis and outcome
The patient tested positive for a concentration of 16 IU/mL of GAD 65 autoantibodies on 3/29/2021, indicating a diagnosis of type 1 diabetes. Likewise, he tested positive for Thyroid Peroxidase antibodies (TPO) at 499 IU/mL, confirming his diagnosis of autoimmune thyroid disease, Hashimoto thyroiditis in this case.
As indicated on Table 2 , the patient’s HbA1c levels rose from 9.1% to 13.5% in about 9 months before being treated with insulin in November 2020. While taking insulin in the form of insulin lispro and insulin degludec, his HbA1c levels dropped from 13.5% to 7.4% in under 4 months. Based on the continuous glucose monitoring (CGM) values between 5/4/21 and 5/17/21, the Glucose Management Indicator (GMI) predicted an HbA1c of 6.4% just 7 months after the first insulin injection. Likewise, his glycemic control improved with a blood glucose 88% in range with no significant lows as of 5/17/21.
HbA1c level evolution
| Date | HbA1c |
|---|---|
| 1/13/20 | 9.1% |
| 7/3/20 | 10.4% |
| 10/29/20 | 13.5% |
| 12/24/20 | 8.8% |
| 2/18/21 | 7.4% |
| 5/4/21-5/17/21 | Expected HbA1c (GMI) = 6.4% |
Discussions
Type 1 and 2 diabetes have clinically distinct pathophysiologic etiologies. These discrete pathologies lead to unique presentations for each type and are useful in informing clinical diagnostic practices as well as treatment regimens. The clinical determination of type 1 vs. 2 diabetes is made with consideration of factors including the patient’s age, body composition, symptom progression and clinical presentation. Type 1 diabetes typically manifests in young patients, often before the age of 14, who frequently appear thin and have a sudden onset of symptoms, with diabetic ketoacidosis presenting as the first sign of disease in many cases. Type 2 diabetes, on the other hand, often develops around the age of 45 or later and is associated with obesity and metabolic syndrome and usually has a gradual onset.
As both types of diabetes generally have distinct etiologies, diagnosis can often be made based on patient demographics. However, atypical cases can exist in contravention of diagnostic norms. The case detailed above discusses one such patient whose clinical characteristics and progression matched closely with the criteria for type 2 diabetes, even though his pathophysiology pointed to type 1 diabetes. While his initial diagnosis of type 2 diabetes was made based on his clinical characteristics, antibody tests significant for glutamic acid decarboxylase 65 autoantibody (GAD 65 ) confirmed his diagnosis with type 1 diabetes [ 3 ]. While rare, similar atypical cases of diabetes have been reported [ 1 , 2 ]. With the increasing number of diabetes cases expected in the coming years [ 4 ], unusual cases such as the case presented above may become more common. To ensure timely and accurate diagnosis and management of patients with atypical cases of diabetes, we seek to raise awareness of cases which do not adhere to presentational or diagnostic norms.
Key Messages
- It is known that atypical cases of diabetes exist and can complicate the diagnosis of diabetes mellitus.
- This manuscript provides evidence that atypical cases can present with symptoms typically associated with another type of diabetes.
- The case described is an example of a type 1 diabetes patient presenting with several classic features of type 2 diabetes.
Conflicts of interest
There are no personal, financial, or other conflicts of interest to disclose.
Consent for publication
Written informed consent from the patient has been taken and is available for review by Editor in chief of the journal.
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
- Katherine Kupfer
- Mackenzie Morris
- Elizabeth Mount
- Chelsea Taylor
- Brittney Wex
Our rationale for choosing this condition:
Throughout our clinical experiences, previous careers, and personal lives, diabetes has been prevalent. As practitioners, we will continue to see this condition often and have to recognize/treat it in our patients. In light of this, we wanted to have a good understanding of Type 1 diabetes, its presentation, and its treatment.
| |||||||||||||||||||||||||||||||||||||||||||||||









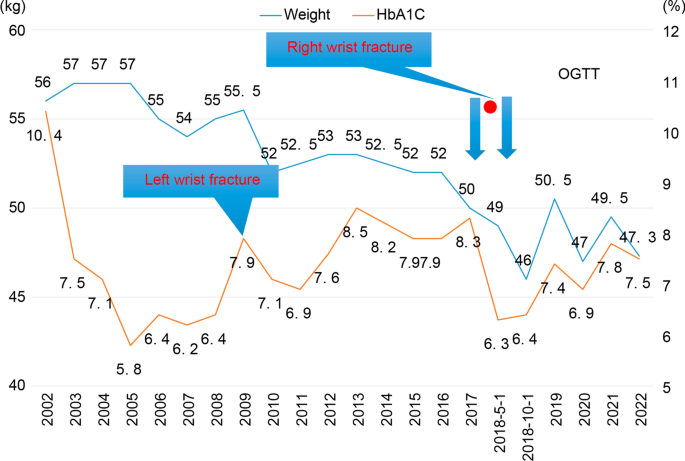
IMAGES
VIDEO
COMMENTS
Registered dietitians (RDs) who have earned the Board Certified-Advanced Diabetes Manager (BC-ADM) credential hold a master's or doctorate degree in a clinically relevant area and have at least 500 hours of recent experience helping with the clinical management of people with diabetes.1 They work in both inpatient and outpatient settings, including diabetes or endocrine-based specialty ...
CASE PRESENTATION. A previously healthy 35‐month‐old girl was brought to the emergency room of the Namazi hospital, Shiraz, Iran, due to reduced level of consciousness. ... First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35‐month‐old girl. Clin Case Rep. 2021; 9:e04984. 10.1002/ccr3.4984 ...
Presentation of Case. Dr. Max C. Petersen (Medicine): A 34-year-old woman was evaluated in the diabetes clinic of this hospital for hyperglycemia. Eleven years before this presentation, the blood ...
A 59-year-old woman with type 1 diabetes and a 2-year history of cognitive decline presented with obtundation. There was diffuse, symmetric hypointensity in the brain on T2-weighted images and abno...
Case 6-2020: A 34-Year-Old Woman with Hyperglycemia
Type 1 Diabetes Mellitus Clinical Presentation
Adult-Onset Type 1 Diabetes: Current Understanding and ...
Approximately 25% of patients that present with DKA have new onset of type 1 diabetes. Antibody testing was not performed, presumably because of the typical type 1 presentation. During his hospital stay, the patient received instructions regarding diet, medication schedule, and home glucose monitoring.
from uptodate: Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents-Authors:Lynne L Levitsky, MDMadhusmita Misra, MD, MPH, updated Oct, 2021; Overview of the management of type 1 diabetes mellitus in children and adolescents ... This wraps up the case study on type one diabetes. Please take a look at ...
Presentation of Case. ... Janež A, Guja C, Mitrakou A, et al. Insulin therapy in adults with type 1 diabetes mellitus: a narrative review. Diabetes Ther 2020;11:387-409. Crossref.
In this new paradigm, a preclinical stage 1 case of type 1 diabetes is defined as the presence of two or more autoantibodies, ... is slightly more common in men and boys than in women and girls. 23 Two HLA class 2 haplotypes involved in anti gen presentation, HLA DRB1*0301-DQA1*0501-DQ*B10201 (DR3) ...
Case 1. An obese 40 year-old woman is admitted for asthma. She received a dose of methylprednisolone in the ED at 7pm and will now be receiving 40 mg of oral prednisone daily in the AM. She had not been on any steroids for many years. Random finger-stick blood glucose when she gets to the floor at 10pm is 275 mg/dL. Her last meal was at 5pm.
Pathophysiology and Clinical Presentation. Pathophysiology: Type 1 Diabetes Mellitus is a syndrome characterized by hyperglycemia and insulin deficiency resulting from the loss of beta cells in pancreatic islets (Mapes & Faulds, 2014). Nonimmune (type 1B diabetes), occurs secondary to other diseases and is much less common than autoimmune (type ...
A case report: First presentation of diabetes mellitus type 1 with severe hyperosmolar hyperglycemic state in a 35-month-old girl Clin Case Rep. 2021 Nov 6;9(11):e04984. doi: 10.1002/ccr3.4984. ... Clinical Case Reports published by John Wiley & Sons Ltd. Publication types
The most recent National Health and Nutrition Examination Survey noted that 30% of adolescents are now overweight, 2 and there has been a commensurate rise in the number of cases of type 2 diabetes found in adolescents. 3 Recently, in a cohort of obese adolescents, 20% were noted to have impaired glucose tolerance, and 4% had undiagnosed type 2 ...
Case study on Diabetes Mellitus | PPT
Our case is an example of a patient whose type 1 diabetes presented atypically with characteristics often associated with type 2 diabetes. Patient presentations such as this are uncommon, with our patient having presented with the "textbook" characteristics of type 2 diabetes. When first diagnosed with diabetes mellitus type 2, the patient ...
11. Introduction Type1 diabetes Insulin Dependent diabetes mellitus, or Juvenile onset DM. Average onset is in childhood or early adulthood (usually before 30 years of age) Due to pancreatic islet destruction predominantly by an autoimmune process. Cell mediated response: - Type 1 diabetes is caused by a T cell mediated autoimmune destruction ...
In light of this, we wanted to have a good understanding of Type 1 diabetes, its presentation, and its treatment. If you have a disability and experience difficulty accessing this content, please email [email protected] or call 614-292-5000 for assistance. The content of this site is published by the site owner (s) and is not a statement of advice ...
Last Update: Updated Chapter 4, Chapter 11 slideset. For comments, corrections or to contribute teaching slides please contact Dr. George Eisenbarth at: [email protected]. Updated Chapter 4 slideset. Banting Slideset (2009) Type 1 Diabetes: Cellular, Molecular & Clinical Immunology Edited by George Eisenbarth. TABLE OF CONTENTS.
Open the PDF Link PDF for Case 3: An Unusual Clinical Presentation of Diabetes Eventually Diagnosed as a Monogenic Form in another window. Case 4: A Case of Monogenic Diabetes. By ... Open the PDF Link PDF for Case 41: New-Onset Type 1 Diabetes, Addison's Disease, and Hypothyroidism: A Case of Autoimmune Polyendocrine Syndrome Type 2 in ...
varshawadnere. This document presents a case study of a 75-year-old patient with diabetes mellitus, chronic asthma, and heart failure. The patient's current medications were interacting and not well-controlled. The presenting doctor assessed the patient and created a new treatment plan including Dapagliflozin, insulin glargine, pantoprazole ...
By actively engaging with these case histories, readers will feel more confident and empowered to manage such presentations effectively in the future. George, a 31-year-old chef, comes to the surgery asking to be tested for diabetes. He reports symptoms of thirst and explains that there is a strong family history of diabetes.
Diabetes-associated antibodies include antibodies to GAD, islet cell antibodies, antibodies to the protein tyrosine-phosphatase-related protein 2 (IA2), and insulin antibodies. 4,6 These autoantibodies have been studied in relatives of patients with type 1 diabetes, and their presence, in the absence of apparent metabolic abnormalities, has a ...
Background Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have diverse effects on sodium and water homeostasis. They decrease thirst perception, potentially inhibit arginine vasopressin (AVP) production, and induce natriuresis. We present three cases of AVP deficiency (AVP-D) where GLP-1 RA initiation led to desmopressin dose reduction. Cases Three patients with AVP-D on stable ...
Background This case report explores the long-term dynamics of insulin secretion and glycemic control in two patients with diabetes mellitus type 2 over 20 years. The observations underscore the impact of lifestyle interventions, including weight loss and calorie restriction, on insulin secretion patterns and glucose levels during 75 g oral glucose tolerance tests. Additionally, the role of ...