

Journals, books & databases
- Our journals
RSC Medicinal Chemistry
The journal for research and review articles in medicinal chemistry and related drug discovery science

You can find details about how to access information remotely in this step-by-step guide . The guide will also help if for any reason you have difficulty accessing the content you want.
What would you like to know about this journal?
RSC Medicinal Chemistry is a Transformative Journal and Plan S compliant
Impact factor: 4.1*
Time to first decision (all decisions): 10.0 days**
Time to first decision (peer reviewed only): 30.0 days***
Editor-in-Chief: Mike Waring
CiteScore: 5.8****
Open access publishing options available
Read this journal
Submit an article
Sign up for regular email alerts
View all journal metrics
Publish open access
Meet the team
Journal scope.
RSC Medicinal Chemistry publishes significant research in medicinal chemistry and related drug discovery science.
Research articles published in this journal must show a breakthrough or significant advance on previously published work, or bring new thinking or results that will have a strong impact in their field.
Examples of areas within the journal's scope are:
- Design, synthesis and biological evaluation of novel chemical entities or biotherapeutic modalities. To be suitable for publication these must exhibit significant potential as new pharmacological agents, tools, probes or potential drugs.
- Modifications of known chemical entities or biotherapeutic modalities that result in a significantly greater understanding of their structure-activity relationships, an improvement of their properties or provide other information of significant value, for example, the identification of a new target or mode of action for a known agent. Routine modifications with minimal or no improvement are not suitable for RSC Medicinal Chemistry .
- Novel methodologies and technologies in the broader chemical and biological sciences (for example, enabling synthetic chemistry, chemical biology, -omics sciences, nanoscience) with application to drug discovery, target identification or elucidation of the mechanism of action. Biological studies should present sufficient innovation with respect to the chemistry.
- Computational studies are welcome where they significantly advance medicinal chemistry knowledge. Studies that use established computational methods should include an original prediction and be accompanied by new experimental data which validates the prediction made. Studies that report novel computational methodology must demonstrate its use in medicinal chemistry through comparison with experimental data. Computational research that does not clearly relate the results obtained to experimental data or that has no demonstrated utility (or where the utility is unlikely to advance the field significantly) is not suitable for RSC Medicinal Chemistry. Docking studies presented without experimental data are not suitable for publication in the journal.
- Studies that examine the effect of the molecular structure of a compound on pharmacokinetic behaviour and pharmacodynamics.
- Studies that present new insights into drug design based on analysis of existing experimental datasets or new theoretical approaches if supported by experimental evidence.
- Studies presenting new drug delivery systems with novel chemical agents are welcomed, in particular those that involve chemical modification of the delivery system of conjugation with novel delivery vectors. Those that focus solely on formulations of known drugs are not suitable for publication in RSC Medicinal Chemistry.
Note that studies where new or existing compounds are tested as pharmacological agents will only be considered if they are carried out in the presence of clear positive and negative controls. Studies of this type should include a clearly defined and hypothesis-driven compound design rationale. Potential antimicrobial agents should be tested for cytotoxicity and activity against non-related pathogens.
To help editors and referees assess the significance of each submitted manuscript we ask all authors on submission to provide a brief statement of significance. This should contain one sentence to summarise the most important finding(s) in the manuscript and a second sentence to say why this is a significant advance in the field. This significance statement should focus specifically on the importance of the piece of research being submitted, rather than the importance of the field.
RSC Medicinal Chemistry Emerging Investigator Lectureship
This Lectureship celebrates outstanding early career researchers who have made significant contributions in the fields of medicinal chemistry and drug discovery. The RSC Medicinal Chemistry Lectureship is awarded annually through a process whereby nominations of candidates are invited from our community.
You can read about eligibility, how to nominate, deadlines for nominations and see all of our lectureship winners.
Find out who is on the editorial and advisory boards for the RSC Medicinal Chemistry journal.
Editor-in-chief
Mike Waring , Newcastle University, UK
Associate editors
Cynthia Dowd , George Washington University, USA
Maria Duca , Université Côte d’Azur - CNRS, France
Sankar K. Guchhait , National Institute of Pharmaceutical Education and Research (NIPER), India
Sally-Ann Poulsen , Griffith University, Queensland, Australia
Jian Zhang , Shanghai Jiao Tong University School of Medicine, China
Editorial board members
Hayley Binch , Hoffman-La Roche, Switzerland
Paola Castaldi , MatchPoint Therapeutics, USA
Lyn Jones , Dana-Farber Cancer Institute, USA
Jean-Louis Reymond , University of Bern, Switzerland
Timor Baasov , Israel Institute of Technology, Israel
Andreas Bender , University of Cambridge, UK
Julian Blagg , Institute of Cancer Research, UK
Margaret Brimble , University of Auckland, New Zealand
Mark Bunnage , Vertex, USA
Christopher Burns , Certa Therapeutics, Australia
Andrea Cavalli , University of Bologna, Italy
Young-Tae Chang , POSTECH, South Korea
James Crawford , Altos Labs, USA
Matthew Duncton , Rigel Pharmaceuticals Inc
Stephen Frye , University of North Carolina at Chapel Hill, USA
Matthew Fuchter , Imperial College London, UK
Sylvie Garneau-Tsodikova , University of Kentucky, USA
Jayanta Haldar , Jawaharlal Nehru Centre for Advanced Scientific Research, India
Gyoonhee Han , Yonsei University, Korea
Mike Hann , GSK Medicines Research Centre, Stevenage, UK
Christian Heinis , EPFL, Switzerland
Laura H. Heitman , Leiden University, Netherlands
Yoshinori Ikeura , Axcelead Drug Discovery Partners, Japan
Ahmed Kamal , NIPER, Hyderabad, India
Robert Langer , MIT, USA
Steven V Ley , University of Cambridge, UK
María Luz López Rodríguez , Complutense University of Madrid, Spain
Christa Muller , University of Bonn, Germany
Roberto Pellicciari , University of Perugia, Italy
David Rees , Astex Therapeutics, Cambridge, UK
Motonari Uesugi , Kyoto University, Japan
John C Vederas , University of Alberta, Canada
Paul Wender , Stanford University, USA
Zhen Yang , Peking University, China
Ming-Qiang Zhang , Amgen, Shanghai, China
Katie Lim , Executive Editor
Harriet Riley , Deputy Editor
Emily Cuffin-Munday , Development Editor
Sarah Anthony , Editorial Production Manager
Nicola Burton , Publishing Editor
Tom Cozens , Publishing Editor
Ryan Kean , Publishing Editor
Roxane Owen , Publishing Editor, ORCID 0000-0002-4553-233X
Lauren Yarrow-Wright , Publishing Editor
Andrea Whiteside , Publishing Assistant
Sam Keltie , Publisher, Journals, ORCID 0000-0002-9369-8414
Article types
RSC Medicinal Chemistry publishes:
- Research articles
- Review articles
Research article
All new research in RSC Medicinal Chemistry is published in the Research article format. Research articles have no page limits, although most articles fall between 4 and 10 journal pages (approximately 10–25 pages of double-spaced text). Research Articles encompass both full paper and communication styles. Where a communication style article is submitted the work should be of enough importance to merit urgent publication before the full study is complete. In all cases authors should provide the same level of experimental detail and data (full details of requirements can be found in the “Journal Specific Guidelines” section below).
Research findings should be presented in an informative way, emphasising the importance and potential impact of the research. Authors should limit experimental procedures and data in the main text to a maximum two journal pages (approximately 5 double-spaced pages), with all additional experimental information and data placed in the electronic supplementary information (ESI).
Authors are particularly encouraged to prepare a title and abstract which concisely summarise the key findings of their research and their importance, avoiding the use of non-standard abbreviations, acronyms and symbols, as this will enable potential readers to quickly understand the significance of the research. Authors should also consider using recognisable, searchable terms, as around 70% of our readers come directly via search engines. The table of contents graphic should give the reader a clear indication of the topic of the study, for example by showing key compounds.
Authors are encouraged to use the article template, available from our Author templates & services page , for preparing their submissions. However, the use of the template for Research article submissions is not essential.
Additional guidance on the layout and formatting of the article and supplementary information can be found on our Prepare your article page .
Review article
These are easy-to-read articles covering current areas of interest for a broad medicinal chemistry audience. They are a concise and critical appraisal of an area in medicinal chemistry or a related topic, typically 6-12 pages in length. We also welcome shorter, mini-review style articles under this article type.
Reviews should focus on the key developments that have shaped the topic, rather than comprehensive reviews of the literature. Authors are encouraged to summarise important findings instead of re-iterating details already available in the primary work and should provide summary figures instead of multiple figures from original manuscripts, where appropriate.
Authors should include their own perspective on developments and trends, and the final paragraphs should discuss future directions, particularly identifying areas where further developments are imminent or that are in urgent need of being addressed.
Please note that Reviews should include balanced coverage of the field and not focus predominantly on the author’s own research.
Opinions are short, personal viewpoints on a topic of current interest to the community. They can be speculative in nature and stimulate counter-opinion, provided that they are not defamatory to the work of others. They should contain rigorous, evidence-backed scientific justification, and bring significant and valuable insights to the field.
Opinions are typically three to four pages in length and are normally published by invitation of the RSC Medicinal Chemistry Editorial Board or Editorial Office. Opinions undergo a rigorous and full peer review procedure, in the same way as Research and Review articles.
Comments and Replies are a medium for the discussion and exchange of scientific opinions between authors and readers concerning material published in RSC Medicinal Chemistry .
For publication, a Comment should present an alternative analysis of and/or new insight into the previously published material. Any Reply should further the discussion presented in the original article and the Comment. Comments and Replies that contain any form of personal attack are not suitable for publication.
Comments that are acceptable for publication will be forwarded to the authors of the work being discussed, and these authors will be given the opportunity to submit a Reply. The Comment and Reply will both be subject to rigorous peer review in consultation with the journal’s Editorial Board where appropriate. The Comment and Reply will be published together.
Transparent peer review
As part of our commitment to transparency and open science, RSC Medicinal Chemistry is now offering authors the option of transparent peer review, where the editor’s decision letter, reviewers’ comments and authors’ response for all versions of the manuscript will be published alongside the article under an Open Access Creative Commons licence (CC-BY) .
Reviewers will remain anonymous unless they choose to sign their report.
Find out more about our transparent peer review policy
Journal specific guidelines
Human and animal welfare.
When a study involves the use of live animals or human subjects, authors must include in the 'methods/experimental' section of the manuscript a statement that all experiments were performed in compliance with the relevant laws and institutional guidelines, and must state the institutional committee(s) that has approved the experiments. A statement that informed consent was obtained for any experimentation with human subjects is required. Reviewers may be asked to comment specifically on any cases in which concerns arise.
More information on the Royal Society of Chemistry journals’ ethical policies can be found in our Author responsibilities page .
Disclosure of chemical structures
Chemical structures should be reported in the manuscript if that structure is necessary to understand the paper or repeat an experimental or computational procedure. Chemical structures should not be blanked out. In certain cases the non-disclosure of chemical structures may be acceptable, and these are considered on a case-by-case basis by the Associate Editor.
Experimental methods and data
Sufficient details of experimental or computational procedures should be included such that a scientist skilled in the art would be able to reproduce the results presented. The synthesis of all new compounds must be described in detail. Descriptions of synthetic procedures must include the specific reagents and solvents employed and must give the amounts (g, mmol) used. Products yields (%) must be reported together with a clear statement of how the percentage yields were calculated. The final physical state (solid; amorphous; liquid; solution) of the product should be disclosed. Where compounds are synthesised as part of an array or library a representative synthesis will be sufficient.
Authors should limit experimental procedures and data to two journal pages (approximately 5 double-spaced pages), with all additional experimental information and data placed in the electronic supplementary information (ESI).
Characterisation of organic compounds
Characterisation levels should be consistent with the importance of the compound to the conclusion of the work:
- For all tested compounds purity should be at least 95%, confirmed by either 1 H/ 13 C NMR data (with spectrum presented in the supplementary file), HPLC, GC, electrophoresis or elemental analysis. Further characterisation data should be supplied where available
- For key compounds (those which are subject to further study beyond initial screening), additional data should include 1 H NMR data (with spectrum presented in the supplementary file) and LC-MS data. Further data such as 13 C NMR, IR, CHN data and HRMS data should be supplied if available
- For chiral compounds, when used as a non-racemate, specific rotation and evidence of enantiomeric purity via chiral HPLC or derivatisation to diastereoisomeric compounds/use of chiral shift reagents should be given. Where HPLC is used conditions employed should be supplied including column type, flow rate, solvent system and detection method
- For compounds made as part of an array that are not considered key compounds, LC-MS data is sufficient.
- For compounds generated through combinatorial methods, lead compounds should be characterised to the same standards as compounds generated through standard synthetic procedures.
- For known compounds, an original reference to previously reported data should be cited; however authors should also include any new, previously unpublished characterisation data that have been obtained for known compounds.
Characterisation of biomolecules (For example, enzymes, peptides, proteins, DNA/RNA, oligosaccharides, oligonucleotides)
Authors should provide evidence for the identity and purity of the biomolecules described. The techniques that may be employed to substantiate identity include the following:
- Mass spectrometry
- Sequencing data (for proteins and oligonucleotides)
- High field 1 H, 13 C NMR
- X-ray crystallography
Purity must be established by one or more of the following:
- Gel electrophoresis
- Capillary electrophoresis
- High field 1 H, 13 C NMR.
Sequence verification should also be provided for nucleic acid cases involving molecular biology. For organic synthesis involving DNA, RNA oligonucleotides, their derivatives or mimics, purity must be established using HPLC and mass spectrometry as a minimum. For new derivatives comprising modified monomers, the usual organic chemistry analytical requirements for the novel monomer must be provided. However, it is not necessary to provide this level of characterisation for the oligonucleotide into which the novel monomer is incorporated.
Novel macromolecular structures and newly reported nucleic acid or protein sequences and microarray data must be deposited with the appropriate database. Articles will not be published until the relevant accession number has been provided. These codes should be quoted in the experimental section of the manuscript. Microarray data should be MIAME compliant.
All Western blot and other electrophoresis data should be supported by the underlying raw images. The image of the full gel and blot, uncropped and unprocessed, should be provided in the supplementary information on submission. All samples and controls used for a comparative analysis should be run on the same gel or blot.
When illustrating the result, any cropping or rearrangement of lanes within an image should be stated in the figure legend and with lane boundaries clearly delineated. Alterations should be kept to a minimum required for clarity.
Each image should be appropriately labelled, with closest molecular mass markers and lanes labelled. All details must be visible, over or underexposed gels and blots are not acceptable. Authors should be able to provide raw data for all replicate experiments upon request.
Biological data
Biological test methods should be described in sufficient detail such that a scientist skilled in the art would be able to reproduce the results presented. Forms of administration as well as physical states and formulations should be noted. Doses and concentrations should be expressed as molar quantities (for example, mol kg -1 , µmol kg -1 , M, µM). For those compounds found to be inactive, the highest concentration ( in vitro ) or dose level ( in vivo ) tested should be indicated. For in vivo studies vehicle information should be supplied.
Quantitative biological data are required for all test compounds. It is expected that all tested compounds would be 95% pure and shown to be so using standard methods. Active compounds from combinatorial syntheses should be re-synthesised and retested to verify biological activity. In these cases experimental procedures and characterisation data as described above should be provided. Known or standard compounds or drugs should be tested under the same experimental conditions for the purpose of comparison (as a positive control). Data may be presented in tabulated form or as graphs; extensive data for compounds should be presented in the electronic supplementary information. Authors should use a number of significant figures that is relevant to the accuracy of the data. Information about the error associated with biological data, for example standard deviation or SEM, should be provided along with the number of experimental determinations.
Pan Assay Interference (PAINS) Compounds
In cases where potential assay interference compounds (for example covalent modifiers, luminescent molecules, redox active compounds, metal chelators, membrane disruptors or unstable compounds which can decompose to form active compounds)are reported as being active, authors should provide evidence in the experimental section that this activity is genuine and is not due to an artefact. For more information about interference compounds see JB Baell and GA Holloway, J. Med. Chem. 2010, 53 , 2719-2740.
Computational studies
Details of the types of computational studies that are suitable for publication in RSC Medicinal Chemistry are given in the “Scope” section above.
Computational methods should be described in sufficient detail such that a scientist skilled in the art would be able to reproduce the results presented. Where computational studies are accompanied by experimental results (for example to validate a prediction) those experimental procedures and data should also be described in detail (see guidelines for experimental procedures above). Where an existing computational method is used authors should provide reasoning why this is appropriate for their study.
QSAR & QSPR studies
Studies which report new methodology or theory should be validated against at least one other common data set for which a study using another method has been published previously. Standard studies must be accompanied by new experimental data which tests their predictive power. To be considered for RSC Medicinal Chemistry such studies should demonstrate significant potential to advance the field of medicinal chemistry. Any data or structures which are used to carry out a QSAR or QSPR study should either be made available as supplementary material, or be freely available elsewhere with a reference to the location included in the manuscript.
Statistical analysis
In articles where there is large-scale statistical analysis one of the named authors should be a statistician.
Guidelines on writing titles, abstracts & table of contents entry
The title, abstract and table of contents entry (graphical abstract) are the first parts of your manuscript that editors, referees and potential readers will see, and once published they play a major part in a researcher’s decision to read your article. Therefore it’s important that these clearly and concisely show the main findings of your research and why they are important.
The title should be short and straightforward to appeal to a general reader, but detailed enough to properly reflect the contents of the article.
- Keep it relatively short – between 8 and 15 words is ideal
- Use easily recognisable words and phrases that can be read quickly
- Use general terms for compounds and procedures rather than specific nomenclature or very specialised terms
- Avoid using non-standard abbreviations and symbols
- Avoid using subjective terms such as “novel”
- Use keywords and familiar, searchable terms – these can increase the chances of your article appearing in search results. Around 70% of our readers come directly via search engines.
The abstract is a single paragraph which summarises the findings of your research. It will help readers to decide whether your article is of interest to them.
- The length can vary from 40 to 150 words, but it should always be concise and easy to read, with recognisable words and phrases.
- It should set out the objectives of the work, the key findings and why this research is important (compared to other research in its field).
- It should emphasise (but not overstate) the significance and potential impact of the research in your article.
- Avoid including detailed information on how the research was carried out. This should be described in the main part of the manuscript.
- Like your title, make sure you use familiar, searchable terms and keywords.
Table of contents entry
A table of contents entry (graphical abstract) is required, which should be submitted at the revision stage. This should include an eye-catching graphic and 1-2 sentence(s) of text to summarise the key findings of the article to the reader. It will appear in the table of contents and feeds – for example, RSS feeds.
The graphic should:
- Be simple, but informative.
- Capture the reader’s attention (the use of colour is encouraged).
- Include a structure, scheme, graph, drawing, photograph or combination that conveys the message of the article. Please note, complex schematics or spectra should be avoided.
- Be original, unpublished artwork created by one of the co-authors. Preferably, the graphic should not be reused and appear again within the article.
- Be suitable for, and uphold the standards of, a scholarly publication that has a global reach.
- Not contain any elements that are offensive or inappropriate, in particular words or images that are discriminatory.
- Not contain large amounts of text. Text should be limited to the labelling of compounds, reaction arrows and diagrams, with long phrases or sentences being avoided. Any text should be clearly legible to a reader.
- Not contain logos, trademarks or brands names.
The text should:
- Be concise and focus only on the key findings of the manuscript and their importance, not the processes used; think about what would grab the attention of the potential reader and would encourage them to read the full article.
- Avoid repeating or paraphrasing the title or abstract.
- Use easily recognisable words and phrases that can be read quickly.
Table of contents specifications:
- The figure should be a maximum size of 8 cm wide x 4 cm high.
- Figures should be supplied as TIFF files, with a resolution of 600 dpi or greater.
- The text supplied should be 1-2 sentences long, using a maximum of 250 characters.
Injectable peptide hydrogels for controlled-release of opioids From DOI: 10.1039/C5MD00440C
Drug trapping in hERG K + channels: (not) a matter of drug size? From DOI: 10.1039/C5MD00443H
Structural hybridization of three aminoglycoside antibiotics yields a potent broad-spectrum bactericide that eludes bacterial resistance enzymes From DOI: 10.1039/C5MD00429B
Rigid amphipathic nucleosides suppress reproduction of the tick-borne encephalitis virus From DOI: 10.1039/C5MD00538H
Vast numbers of prevalent aminoglycoside-modifying enzymes undermine the clinical use of aminoglycoside antibiotics. We present the design and synthesis of a potent broad-spectrum bactericidal aminoglycoside based on available X-ray co-crystal structures within the ribosomal binding-site. The resulting antibiotic displays broad protection of its functional groups from inactivation by clinically relevant resistance enzymes.
From DOI: 10.1039/C5MD00429B
Advanced glycation end products (AGEs) are associated with various diseases, especially during aging and the development of diabetes and uremia. To better understand these biological processes, investigation of the in vivo kinetics of AGEs, i.e., analysis of trafficking and clearance properties, was carried out by molecular imaging. Following the preparation of Cy7.5-labeled AGE-albumin and intravenous injection in BALB/cA-nu/nu mice, noninvasive fluorescence kinetics analysis was performed. In vivo imaging and fluorescence microscopy analysis revealed that non-enzymatic AGEs were smoothly captured by scavenger cells in the liver, i.e., Kupffer and other sinusoidal cells, but were unable to be properly cleared from the body. Overall, these results highlight an important link between AGEs and various disorders
From DOI: 10.1039/C6OB00098C
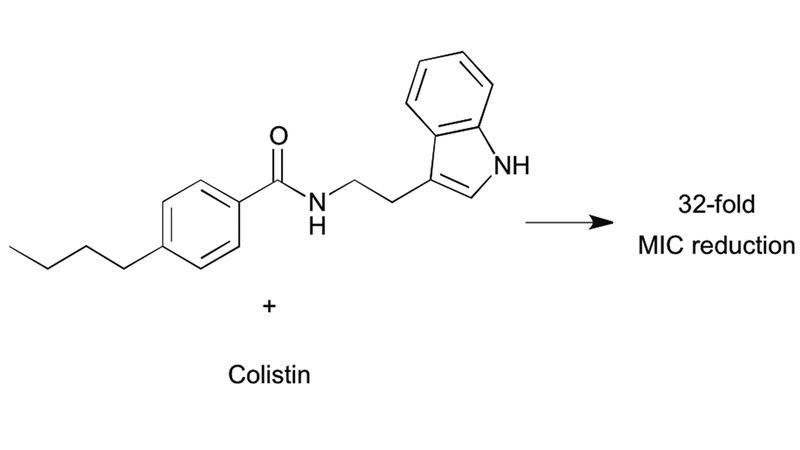
A screen of 20 compounds identified small molecule adjuvants capable of potentiating antibiotic activity against Francisella philomiragia . Analogue synthesis of an initial hit compound led to the discovery of a potentially new class of small molecule adjuvants containing an indole core. The lead compound was able to lower the MIC of colistin by 32-fold against intrinsically resistant F. philomiragia .
From DOI: 10.1039/C5MD00353A
Table of contents
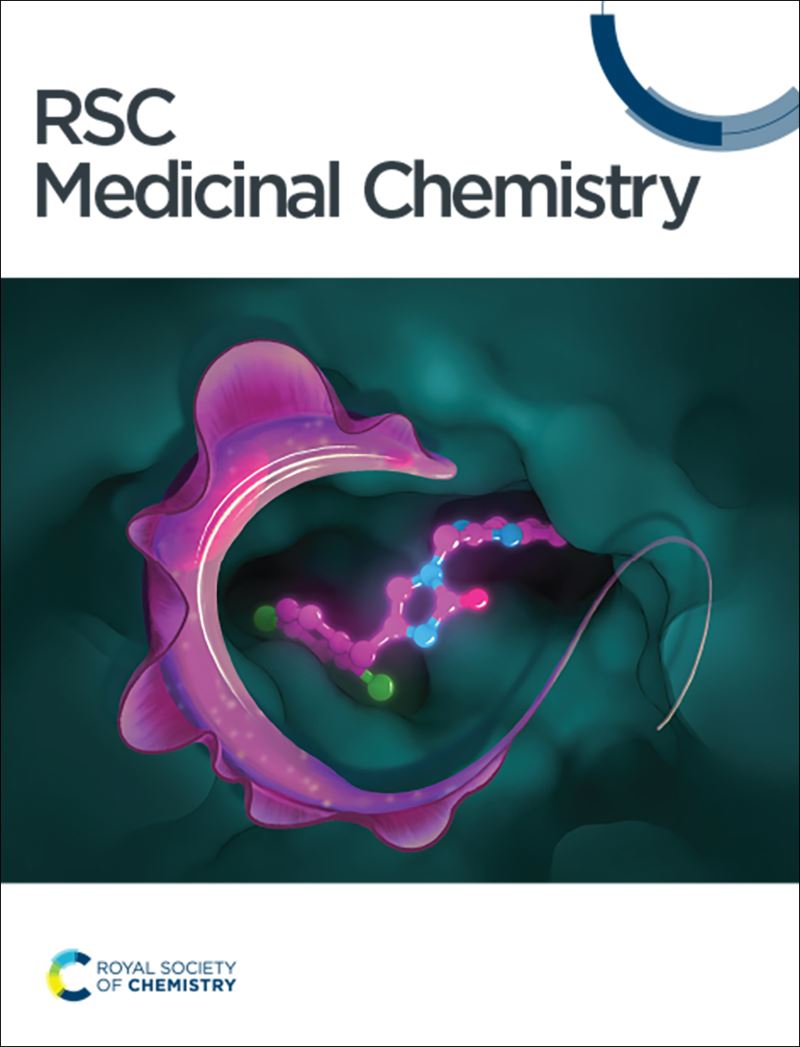
Structural modifications through bioisosteric approach yielded fusidic acid analogues with 2–35 folds increase in antiplasmodial activity as compared to fusidic acid. From DOI: 10.1039/C5MD00343A
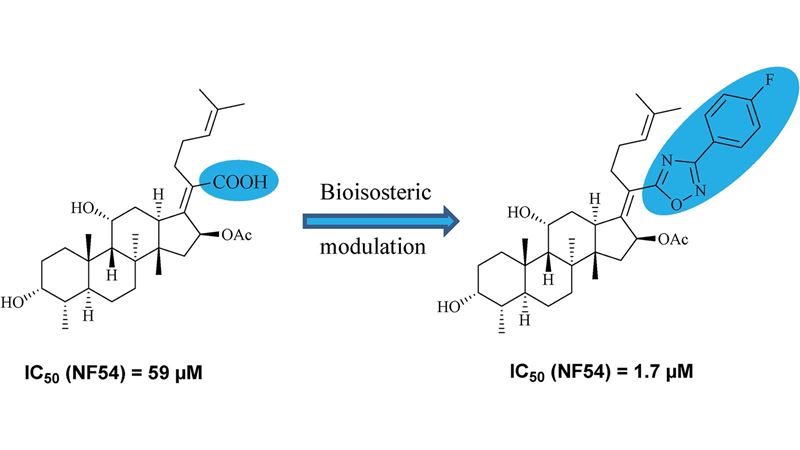
The combination of flow chemistry and computational tools has been successfully applied to prepare a focused library of tricyclic tetrahydroquinolines endowed with drug-like properties. From DOI: 10.1039/C5MD00455A
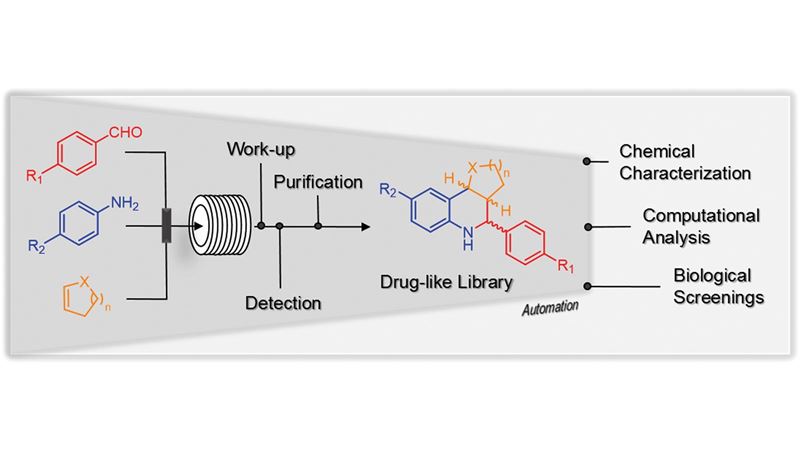
A screen of 20 compounds identified small molecule adjuvants capable of potentiating antibiotic activity against Francisella philomiragia . From DOI: 10.1039/C5MD00353A
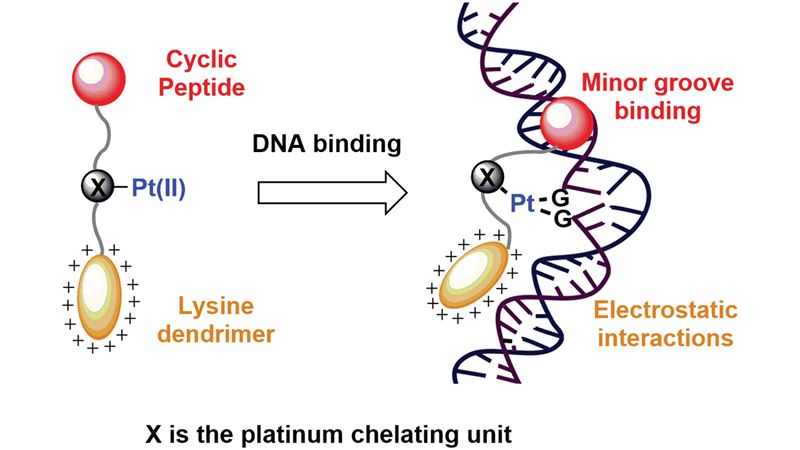
A platinum complex/peptide chimera shows specific DNA binding and covalent platination with potential as a novel chemotherapeutic. From DOI: 10.1039/C5OB01885D
Open access publishing options
RSC Medicinal Chemistry is a hybrid (transformative) journal and gives authors the choice of publishing their research either via the traditional subscription-based model or instead by choosing our gold open access option. Find out more about our Transformative Journals. which are Plan S compliant .
Gold open access
For authors who want to publish their article gold open access , RSC Medicinal Chemistry charges an article processing charge (APC) of £2,750 (+ any applicable tax). Our APC is all-inclusive and makes your article freely available online immediately, permanently, and includes your choice of Creative Commons licence (CC BY or CC BY-NC) at no extra cost. It is not a submission charge, so you only pay if your article is accepted for publication.
Learn more about publishing open access .
Read & Publish
If your institution has a Read & Publish agreement in place with the Royal Society of Chemistry, APCs for gold open access publishing in RSC Medicinal Chemistry may already be covered.
Use our journal finder to check if your institution has an open access agreement with us.
Please use your official institutional email address to submit your manuscript and check you are assigned as the corresponding author; this helps us to identify if you are eligible for Read & Publish or other APC discounts.
Traditional subscription model
Authors can also publish in RSC Medicinal Chemistry via the traditional subscription model without needing to pay an APC. Articles published via this route are available to institutions and individuals who subscribe to the journal. Our standard licence allows you to make the accepted manuscript of your article freely available after a 12-month embargo period. This is known as the green route to open access.
Learn more about green open access .
Readership information
Researchers in academia and industry studying medicinal chemistry, pharmacology, and topics in the wider chemical, biological and materials sciences with application to biological problems.
Subscription information
RSC Medicinal Chemistry is part of the RSC Gold subscription package.
Online only 2024 : ISSN 2632-8682, £1,709 / $2,533
*2023 Journal Citation Reports (Clarivate Analytics, 2024)
**The median time from submission to first decision including manuscripts rejected without peer review from the previous calendar year
***The median time from submission to first decision for peer-reviewed manuscripts from the previous calendar year
****CiteScore™ 2023 available at www.scopus.com/sources

Advertisement
MEDICINAL CHEMISTRY: SCOPE, APPLICATIONS, AND SIGNIFICANCE IN MODERN SCIENCE MARVELLOUS EYUBE
- International Journal of Innovative Research in Computer Science & Technology Volume 9(Issue 5):1
- Volume 9(Issue 5):1

- University of Benin
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Walter Sneader
- Joseph A. DiMasi

- NEW ENGL J MED
- Francis S. Collins

- Graham L Patrick
- CHEM SOC REV
- Paul T. Anastas
- Nicolas Eghbali
- BRIT J PHARMACOL

- R. W. Langer
- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Open access
- Published: 19 December 2018
Therapeutic importance of synthetic thiophene
- Rashmi Shah 1 &
- Prabhakar Kumar Verma 1
Chemistry Central Journal volume 12 , Article number: 137 ( 2018 ) Cite this article
20k Accesses
157 Citations
3 Altmetric
Metrics details
Thiophene and its substituted derivatives are very important class of heterocyclic compounds which shows interesting applications in the field of medicinal chemistry. It has made an indispensable anchor for medicinal chemists to produce combinatorial library and carry out exhaustive efforts in the search of lead molecules. It has been reported to possess a wide range of therapeutic properties with diverse applications in medicinal chemistry and material science, attracting great interest in industry as well as academia. It has been proven to be effectual drugs in present respective disease scenario. They are remarkably effective compounds both with respect to their biological and physiological functions such as anti-inflammatory, anti-psychotic, anti-arrhythmic, anti-anxiety, anti-fungal, antioxidant, estrogen receptor modulating, anti-mitotic, anti-microbial, kinases inhibiting and anti-cancer. Thus the synthesis and characterization of novel thiophene moieties with wider therapeutic activity is a topic of interest for the medicinal chemist to synthesize and investigate new structural prototypes with more effective pharmacological activity. However, several commercially available drugs such as Tipepidine, Tiquizium Bromides, Timepidium Bromide, Dorzolamide, Tioconazole, Citizolam, Sertaconazole Nitrate and Benocyclidine also contain thiophene nucleus. Therefore, it seems to be a requirement to collect recent information in order to understand the current status of the thiophene nucleus in medicinal chemistry research.
Introduction
As the world’s population is increasing at an alarming rate, health problems have also become a very serious clinical problem. Therefore, it is an urgent requirement for the scientist to design and discover new drug molecules which possibly offers some of the greatest hopes for success in present and future epoch. However, there are still enormous numbers of pharmacologically active heterocyclic compounds which are in regular clinical use [ 1 ]. Heterocyclic compounds are extensively distributed in nature and have versatile synthetic applicability and biological activity which helped the medicinal chemist to plan, organize and implement new approaches towards the discovery of novel drugs [ 2 ].
Thiophene (Fig. 1 ) is a five membered heteroaromatic compound containing a sulfur atom at 1 position. It is considered to be a structural alert with formula C 4 H 4 S, chemical name is thiacyclopentadiene [ 3 ].
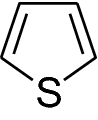
Thiophene was discovered as a contaminant in benzene [ 4 ]. It has the molecular mass of 84.14 g/mol, density is 1.051 g/ml and Melting Point is − 38 °C. It is soluble in most organic solvents like alcohol and ether but insoluble in water. The “electron pairs” on sulfur are significantly delocalized in the π electron system and behaves extremely reactive like benzene derivative. Thiophene forms a azeotrope with ethanol like benzene. The similarity between the physicochemical properties of benzene and thiophene is remarkable. For example, the boiling point of benzene is 81.1 °C and that of thiophene is 84.4 °C (at 760 mmHg) and therefore, both are a well known example of bioisosterism [ 5 ]. It can be easily sulfonated, nitrated, halogenated, acylated but cannot be alkylated and oxidized [ 3 ].
In medicinal chemistry, thiophene derivatives are very important heterocycles exhibiting remarkable applications in different disciplines. In medicine, thiophene derivatives shows antimicrobial [ 6 ], analgesic and anti-inflammatory [ 7 ], antihypertensive [ 8 ], and antitumor activity [ 9 ] while they are also used as inhibitors of corrosion of metals [ 10 ] or in the fabrication of light-emitting diodes in material science [ 11 ].
Biological activities of thiophene derivatives
Thiophene nucleus containing compounds show various activities like for example 1-[1-(2,5-dimethylthiophen-3-yl)ethyl]-1-hydroxyurea ( 1 ) act as an anti-inflammatory agent; the maleate salt of 1-(2,5-dimethylthiophen-3-yl)-3-(5-methyl-1 H -imidazol-4-yl)propan-1-one ( 2 ) work as serotonin antagonists and is used in the treatment of Alzheimer’s disease.
|
|
2-Butylthiophene ( 3 ) is used as a raw material in the synthesis of anticancer agents and 2-octylthiophene ( 4 ) is used in the synthesis of anti-atherosclerotic agents such as ( 5 ). It also act as metal complexing agents and in the development of insecticides.
The higher alkylated thiophenes ( 6 ) has been used extensively as a raw material in patents relating to liquid crystals [ 12 ].
Antimicrobial activity
Thiophene derivatives show high antimicrobial activity against various microbial infections. Different approaches were made to prove thiophene as antimicrobial agent by different scientist for the discovery of most active thiophene derivatives to the present scenario [ 13 ].
Mehta et al. [ 14 ] developed a new class of 4-(1-(3-chlorobenzo[ b ]thiophene-2-carbonyl)-1 H -indol-3-yl)-7, 7-dimethyl-3,4,7,8-tetrahydroquinazoline 2,5(1 H ,6 H )dione thiophene derivatives (Scheme 1 ). These synthesized compounds were screened for their antibacterial activity against three bacterial strains viz. E. coli, P. aeruginosa, S. aureus and three fungal strains viz. C. albicans, A. niger, A. Clavatus using serial broth dilution method. The standard drug used in this study was ‘Ampicillin’ for evaluating antibacterial activity which showed (50, 100, and 50 μg/ml) MIC against E. coli, P. aeruginosa and S. aureus, respectively. For antifungal activity ‘Griseofulvin’ was used as a standard drug, which showed (100, 100, and 100 μg/ml) MIC against C. albicans, A. niger , and A. clavatus , respectively. Among the synthesized derivatives, Compound 4 was found to be good active against P. aeruginosa. For the antifungal activity compounds 4 was considered as good active against A. niger and A. clavatus. The results of synthesized compounds presented in Table 1 .
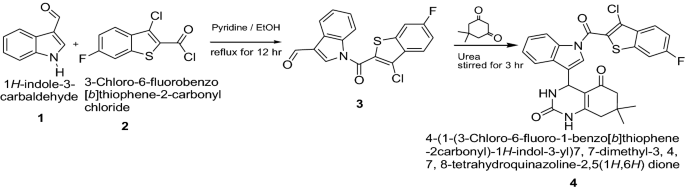
Synthesis of 4-(1-(3-chloro-6-fluoro-1-benzo[ b ]thiophene-2-carbonyl)-1 H -indol-3-yl)-7,7-dimethyl-3,4,7,8-tetrahydroquinazoline 2,5(1 H ,6 H )dione
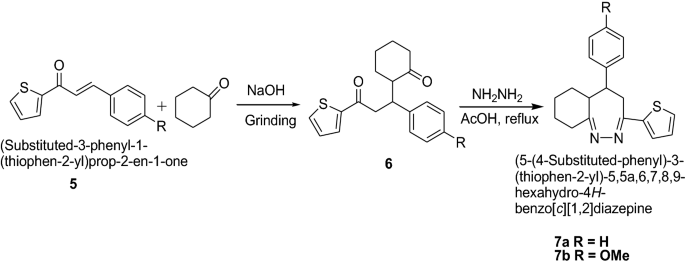
Mazimba [ 15 ] synthesized thiophene analogues of chalcones in good yields by condensation of 2-acetylthiophene and salicylaldehydes using Scheme 2 . 1,5-Diketones were formed by solvent-free michael addition of cyclohexanone and 2-thienylchalcones devoid of hydroxyl groups which were used as synthons for synthesis of diazepines. The synthesized compounds were screened for in vitro antimicrobial activities against S. aureus , E. coli , B. subtilis , P. Aeruginosa and C. Albicans using dilution method. The compounds were found to show moderate to good antibacterial and antifungal activities. Among the tested compounds, diazepines ( 7a , b ) exhibited excellent antibacterial ( S. aureus and P. aeruginosa ) and antifungal ( C. albicans ) activities. The results showed the importance of the carbon–nitrogen bond in biological systems because of which antimicrobial activities for these N-containing compounds were reported. The results of synthesized compounds showed in Table 2 .
Synthesis of diazepines ( 7a , 7b )
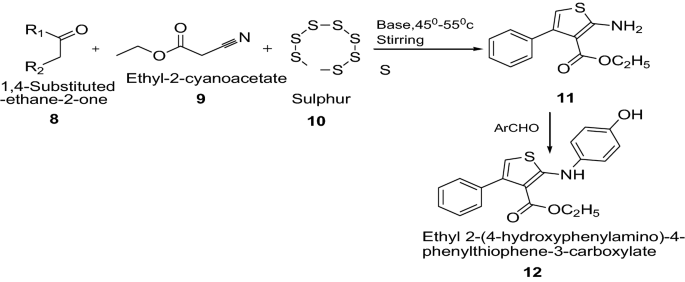
Prasad et al. [ 16 ] synthesized newly ethyl 2-amino-4-phenylthiophene-3-carboxylate derivatives using Scheme 3 . The synthesized compounds were screened for their antibacterial activity by using minimum inhibitory concentration (MIC) method by taking ampicillin and streptomycin as standard drug. Among all the synthesized derivatives, compound 12 showed greater inhibitory effect against the organisms used, particularly against B. subtilis , E. coli , P. vulgaris and S. aureus with MIC. The present study has given deep insight as the 2-aminothiophene bearing 4-hydroxy benzaldehyde shown significant anti-microbial activity. The compound 12 showed the significant anti-microbial activity among all the synthesized 2-aminothiophene derivatives because of the presence of 4-hydroxy benzaldehyde at second position. The results of synthesized compounds presented in Table 3 .
Synthesis of ethyl 2-(4-hydroxyphenylamino)-4-phenylthiophene-3-carboxylate
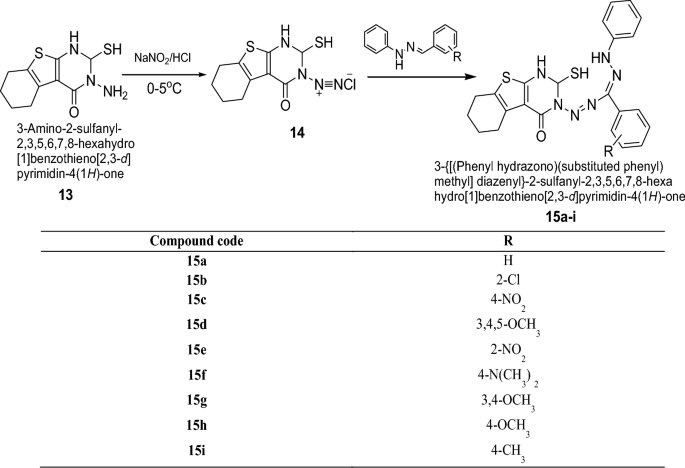
Lakshmi et al. [ 17 ] synthesized 3-{[(phenylhydrazono) (substituted phenyl)methyl]diazenyl}-2-sulfanyl-2,3,5,6,7,8-hexahydro [ 1 ] benzothieno[2,3- d ]pyrimidin-4(1 H )-one derivatives by using Scheme 4 . All the synthesized compounds were screened for their antibacterial and antifungal activities against various microbes such as B. subtilis , E. coli, P. aeruginosa and C. albicans by the cup-plate agar diffusion method. From all the series, compounds 15a , 15c , 15g , 15h , 15i were active against B. subtilis , compounds 15b , 15d , 15e , 15h , 15i were active against E. coli , compounds 15a , 15c , 15d , 15e , 15g , 15h , 15i showed activity against P. aeruginosa and compounds 15a , 15b , 15c , 15f , 15g , 15h , 15i were found active against C. albicans . The results of synthesized compounds showed in Table 4 .
Synthesis of 3-{[(phenylhydrazono)(substitutedphenyl)methyl]diazenyl}-2-sulfanyl-2,3,5,6,7,8-hexahydro [ 1 ] benzothieno[2,3- d ]pyrimidin-4(1 H )-one ( 15a – i )
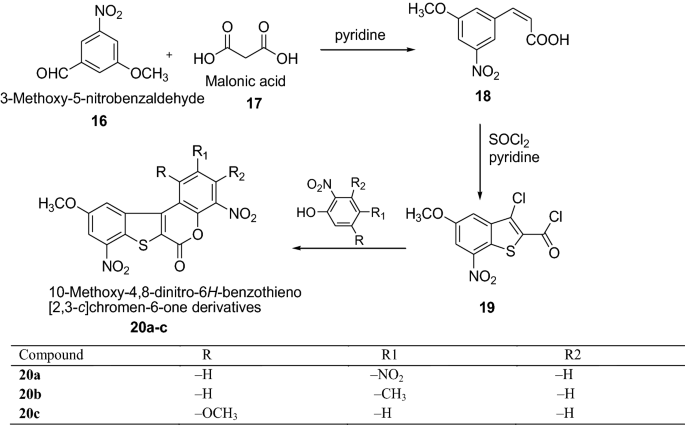
Havaldar et al. [ 18 ] synthesized 10-methoxy-4,8-dinitro-6 H -benzothieno[2,3- c ]chromen-6-one derivatives by using Scheme 5 . All the synthesized compounds were tested for their antibacterial activity against S. aureus , E. coli , B. subtilis and S. typhosa using concentrations of 2 and 5 µg/ml by the ditch plate technique. Among all the series, the compounds 20b showed a much higher inhibitory effect on the growth of bacteria because of the presence of CH 3 group. The results of synthesized compounds presented in Table 5 .
Synthesis of 10-methoxy-4,8-dinitro-6 H -benzothieno[2,3- c ]chromen-6-one derivatives ( 20a – c )
Ahmed et al. [ 19 ] synthesized thieno[3,2- b ]pyridine-2-one derivatives by using Scheme 6 . The synthesized thienopyridines derivatives were evaluated for their in vitro antibacterial activity against two grampositive ( B. subtilis and S. aureus ) and two Gram-negative ( E. coli and S. typhi ) strains using paper disk diffusion assay method by comparing with amoxicillin (30 μg/disk) as reference antibiotic. The compounds 25a and 25b showed remarkable biological activity because of the substitution of the CN (at C3) either by acetyl (as in 25a ) and/or ethoxycarbonyl (as in 25b ). However, the antibacterial activity was slightly hampered by the existence of the electron withdrawing p -bromophenyl group at fourth position of carbon. The results of synthesized compounds presented in Table 6 .
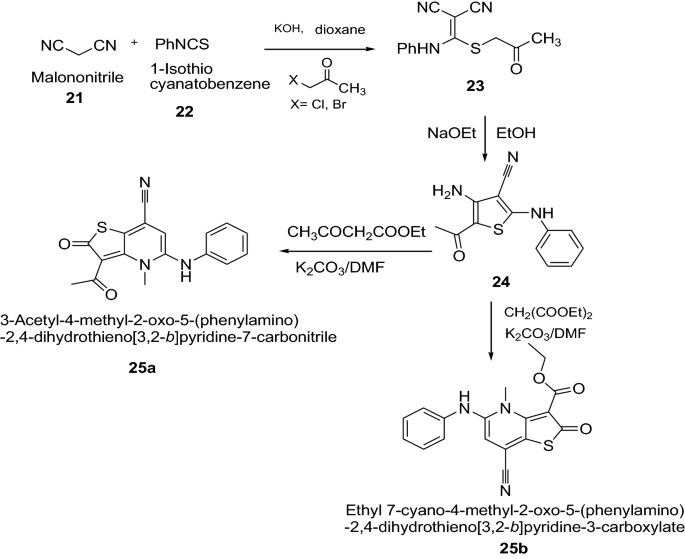
Synthesis of Ethyl 7-cyano-4-methyl-2-oxo-5-(phenylamino)-1,2-dihydrothieno[3,2- b ]pyridine
Bhuiyan et al. [ 20 ] synthesized a novel class of [1,2,4]triazolo[4,3- c ]thieno-[3,2- e ] pyrimidine derivatives using Scheme 7 and assayed for the antibacterial activity against B. cereus , S. dysenteriae and S. typhi and for antifungal activity against M. phaseolina , F. equiseti , A. alternate and C. corchori . The disc diffusion method and poisoned-food techniques were used for antibacterial and antifungal activities, respectively. Among the synthesized compounds 28 and 33 resulted in wide spectrum antimicrobial activity against all the test bacteria and fungi using ampicillin and nystatin as a standard drug, respectively. Introduction of imidazo ( 28 ) or pyrazolo ( 33 ) moiety to the pyrimidine derivatives might be responsible for enhancement of antimicrobial activity of these compounds. The results of synthesized compounds are presented in Tables 7 and 8 .
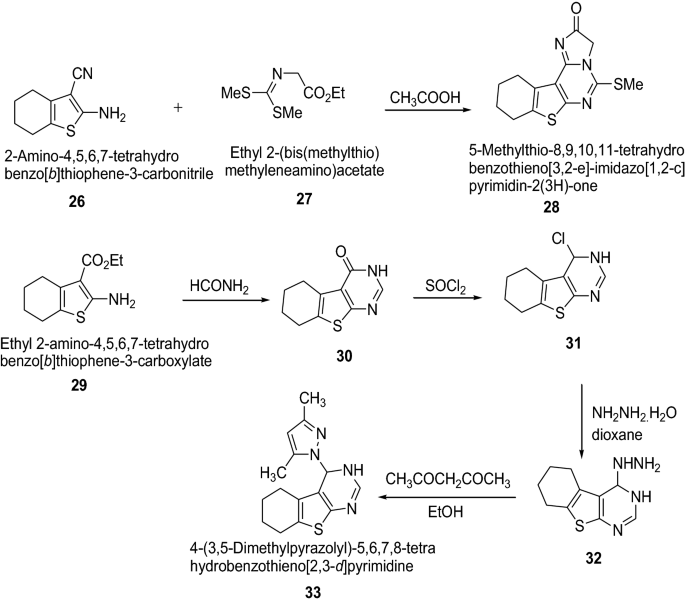
Synthesis of thienopyrimidine derivatives
Khazi et al. [ 21 ] developed some novel tricyclic thienopyrimidines and triazole fused tetracyclic thienopyrimidines derivatives by employing the Gewald reaction (Scheme 8 ). The synthesized compounds were evaluated against two Gram positive bacteria ( S. aureus , B. subtilis ), two Gram negative bacteria ( P. aeruginosa , E. coli ) and two yeast-like fungi C. albicans and C. parapsilosis using the broth micro dilution method. The result indicated that the compounds 35 , 37 , 39a , 39b and 39c have exhibited good antibacterial activity against B. subtilis comparable to the standard ampicillin, while compound 38 displayed better antifungal activity against C. albicans comparable to the standard fluconazole. The results of synthesized compounds are presented in Table 9 .
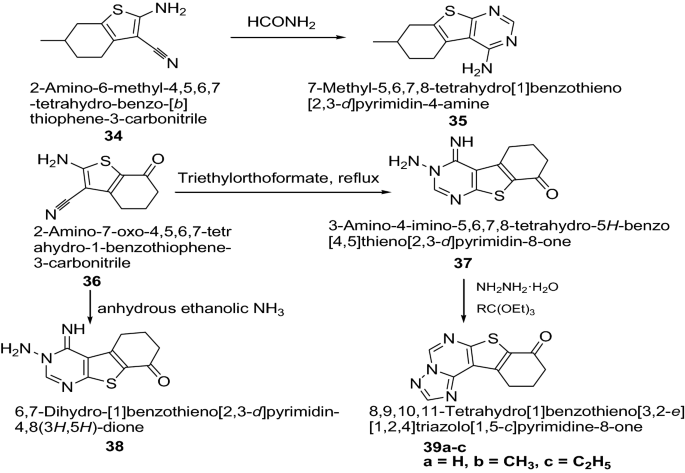
Synthesis of thienopyrimidines and triazolothienopyrimidines derivatives
Tombary et al. [ 22 ] synthesized series of tetrahydrobenzothieno[2,3- d ]pyrimidine and tetrahydrobenzothienotriazolopyrimidine derivatives as presented in Scheme 9 and evaluated for their antimicrobial activity using the cup diffusion technique against S. aureus as Gram-positive bacteria, E. coli and P. aeruginosa as Gram-negative bacteria in addition to C. albicans as fungi. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) for the active compounds were studied and compared with ampicillin and clotrimazole as reference antibiotics. Antimicrobial testing revealed that compounds 44a and 47 were the most active among the tested compounds against C. albicans while compounds 44b and 46 showed the highest antibacterial potency against P. aeruginosa among the tested compounds. The significant results of these compounds are presented in Table 10 .

Synthesis of tetrahydrobenzothieno[2,3- d ]pyrimidine and tetrahydrobenzothienotri azolopyrimidine
Adiwish et al. [ 23 ] synthesized tetra substituted thiophenes from ketene dithioacetals as represented in Scheme 10 . The synthesized compounds 49a and 49b were evaluated in vitro for their antibacterial activity against Gram-positive bacteria ( S. aureus and B. subtilis ) and Gram-negative bacteria ( E. coli and K. pneumonia ) by using agar disc-diffusion technique. The result revealed that compound 49a exhibited bigger inhibition zones compared to 49b . The results of synthesized compounds presented in Table 11 .
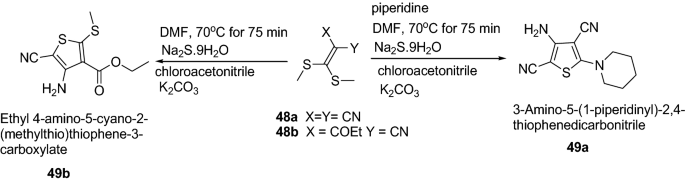
Synthesis of tetrasubstituted thiophenes derivatives
Reheim et al. [ 24 ] synthesized some novel substituted thieno[3,2- c ]pyrazole and pyrazolo[3′,4′:4,5]thieno[2,3- d ]pyrimidine derivatives as represented in Scheme 11 . The antimicrobial activity of the target synthesized compounds were screened against various microorganisms such as E. coli , B. megaterium , B. subtilis , F. proliferatum , T. harzianum , A. niger by the disc diffusion method. Antibacterial activity result indicated that among the synthesized derivatives, compounds 51 , 54 and 56 showed promising broad spectrum antibacterial activities against E. coli . The results of synthesized compounds presented in Table 12 .
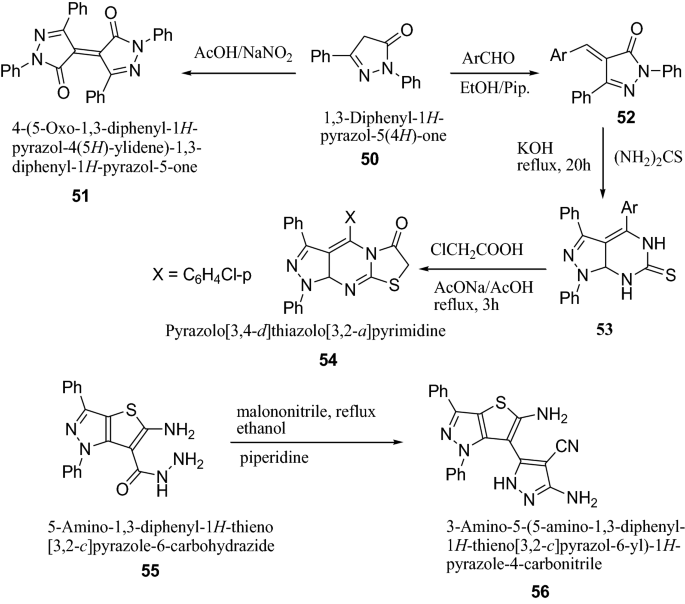
Synthesis of substituted thieno[3,2- c ]pyrazole and pyrazolo[3′,4′:4,5]thieno[2,3- d ]pyrimidine derivatives
Anticancer activity
Cancer is among the most challenging health problems worldwide which has become a major problem for increasing mortality rate globally. Currently available treatments such as chemotherapy and radiotherapy can only provide temporary therapeutic benefits as well as being limited by a narrow therapeutic index, remarkable toxicity, and acquired resistance for most of the type of cancer. However, the research of anticancer drugs in the past several decades has shown extensive progress and has cured considerable number of patients. Still it is the extreme area of investigation due to the complex physiological changes in the cell functionality, metastasis and apoptotic mechanisms. Lots of compounds were screened for anticancer activity in the past few years because of the presence of various cell lines and screening methods. Most of the scientist has synthesized and investigated some of novel thiophene derivatives for the anticancer activity carrying the biologically active sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties [ 25 , 26 , 27 ].
Ghorab et al. [ 28 ] developed a novel series of thiophenes derivatives having biologically active sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties as presented in Scheme 12 . The synthesized compounds were evaluated for in vitro anticancer activity against human breast cancer cell line (MCF7). Many of them showed cytotoxic activities compared to doxorubicin as a positive control. Among this series, ( Z )-4-(3-oxo-3-(thiophen-2-yl)prop-1-enylamino)- N -(thiazol-2-yl)benzenesulfonamide ( 59 ), ( Z )-4-(3-oxo-3-(thio-phen-2-yl)prop-1-enylamino)- N -(1-phenyl-1 H -pyrazol-5-yl)benzenesulfonamide ( 60 ), ( Z )-4-(3-oxo-3-(thiophen-2-yl)prop-1-enylamino)- N -(pyrimidin-2-yl)benzenesulfonamide ( 61 ) and ( Z )-3-(4 methoxybenzo[ d ]thiazol-2-ylamino)-1-(thiophen-2-yl)prop-2-en-1-one ( 62 ) having IC 50 values 10.25, 9.70, 9.55 and 9.39 μmol/l, respectively revealed a promising anti-breast cancer activity than that of doxorubicin with IC 50 = 32.00 μmol/l. It was mainly due to the thiophene nucleus containing biologically active sulfathiazole 59 , sulfaphenazole 60 , sulfadiazine 61 , or benzothiazole 62 moieties. The results of synthesized compounds showed in Table 13 .
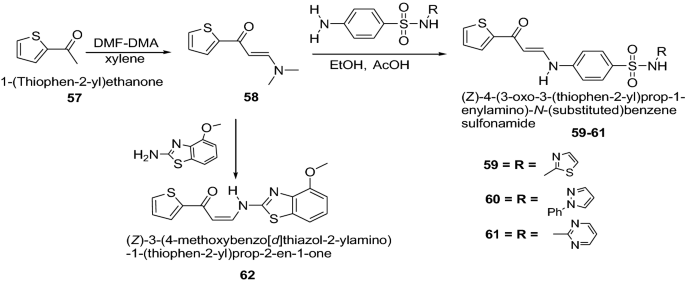
Synthesis of thiophenes having the biologically active sulfonamide ( 59 – 61 ) and 3-methylisoxazole 12,4-methoxybenzo[ d ]thiazole ( 62 )
Gaunda et al. [ 29 ] synthesized some new derivatives of 3-[(2-substituted-6,7,8,9-tetrahydro-5 H -cyclohepta[ b ]thieno[2,3- d ]pyrimidin-4-yl)amino]propan-1-ol derivatives (Scheme 13 ). The in vitro cytotoxicity activity of synthesized compounds were screened against both the cell lines (HC 29-Colorectal adenoma cell line and MDA 231-adenocarcinoma breast cancer cell line) by MTT assay and analyzed statistically. Among this series, the compound 69c had shown better anticancer activity at all concentrations on both the cell lines followed by compound 69a , 69b . It was due to the phenyl substitution ( 69c ) which has shown better anticancer activity. However, all the synthesized compounds showed considerable anticancer activity as compared to cyclophosphamide. The results of synthesized compounds presented in Table 14 .
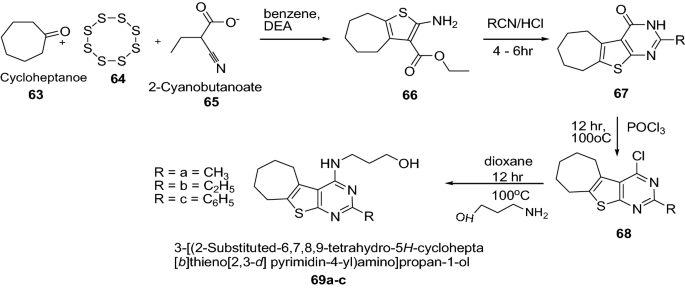
Synthesis of 3-[(2-substituted-6,7,8,9-tetrahydro-5 H -cyclohepta[ b ]thieno[2,3- d ]pyrimidin-4-yl)amino]propan-1-ol
Mohareb et al. [ 30 ] developed a convenient synthetic approach for novel thiophene and benzothiophene derivatives (Scheme 14 ). The in vitro cytotoxicity was screened against three tumor cell lines–MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer) and SF-268 (CNS cancer) and a normal fibroblast human cell line (WI-38) compared to the anti-proliferative effects of the reference control doxorubicin. Among the series, ethyl-5-amino-3-(4-chlorostyryl)-4-cyanothiophene-2-carboxylate ( 74 ), ethyl 5-amino-4-[(4-methoxyphenyl)carbamoyl]-3-methylthiophene-2-carboxylate ( 76b ) and ethyl 5-(3-ethoxy-3-oxopropanamido)-3-methyl-4-(phenylcarbamoyl)thiophene-2-carboxylate ( 77 ) were found to be the most active compounds against the three tumor cell lines such as MCF-7, NCI-H460 and SF-268 where as they showed low potency against the normal fibroblasts human cell line (WI-38). It was revealed that higher cytotoxicity activity of compound 74 was due to the presence of the chloro group, OCH 3 group in compound 76b and the presence of two ethoxy groups in compound 77 . Thus it has been shown that, in most cases, the electronegative Cl, OCH 3 and OC 2 H 5 hydrophobic groups in the thiophene derivatives might play a very important role in enhancing the cytotoxic effect. The results of synthesized compounds presented in Table 15 .
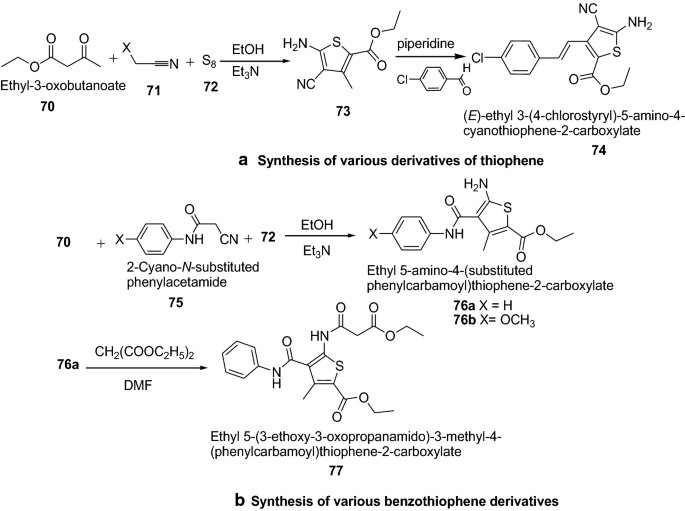
a Synthesis of various derivatives of thiophene, b Synthesis of various benzothiophene derivatives
Sharkawy et al. [ 31 ] synthesized a series of thiophene incorporating pyrazolone moieties via diazo coupling of diazonium salt of 3-substituted-2-amino-4,5,6,7-tetrahydrobenzo[ b ]thiophenes with 3-methyl-1 H -pyrazol-5(4 H )-one, 3-methyl-1-phenyl-1 H -pyrazol-5(4 H )-one or 3-amino-1 H -pyrazol-5(4 H )-one, respectively as represented in Scheme 15 . Newly synthesized derivatives were tested for cytotoxicity against the well known established model ehrlich ascites carcinoma cells (EAC) in vitro. The results showed clearly that compounds 80a – c exhibited high cytotoxic activity than 5-fluorouracil which may be due to the presence of amino group in position 3 of the pyrazol-5-one moiety. Further, the order of antitumor activity of this series of synthesized compounds follows 80c < 80b < 80a which may be due to replacement of CONH 2 by CN or COOC 2 H 5 groups of benzothiophene ring in position 3. The results of synthesized compounds showed in Table 16 .
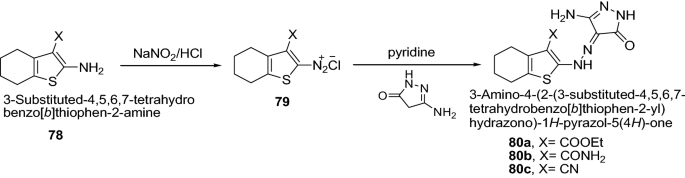
Synthesis of substituted-4-{2-[(or 3-phenyl-)4,5,6,7-tetrahydrobenzo[ b ]thiophen-2-yl]hydrazono}-1 H -pyrazol-5(4 H )-one derivatives
Seley et al. [ 32 ] synthesized tricyclic thieno-separated purine analogues using Scheme 16 . These synthesized derivatives were screened for their cytotoxic activity against HCT116 colorectal cancer cell lines. In this series, compound 83 showed potent cytotoxic activity against cancer cell lines. It was due to the coupling of compound 83 to a ribo-sugar to create the thieno-separated nucleosides may increase the growth inhibitory properties of these analogues. The results of synthesized compounds presented in Table 17 .
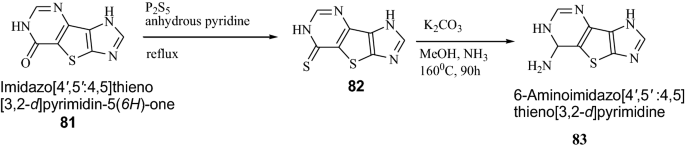
Synthesis of 6-Aminoimidazo[4′,5′:4,5]thieno[3,2- d ]pyrimidine
Mohareb et al. [ 33 ] synthesized novel heterocyclic compounds from 2-cyano- N -(3-cyano-4,5,6,7-tetrahydrobenzo[ b ]thiophen-2-yl)-acetamide as presented in Scheme 17 . The tumor cell growth inhibition activities of the newly synthesized thiophene systems were assessed in vitro on three human tumor cell lines, namely, MCF-7 (breast adenocarcinoma), NCI-H460 (non-small cell lung cancer), and SF-268 (CNS cancer) after a continuous exposure of 48 h. The results were compared to the antiproliferative effects of the reference control doxorubicin. In this series, compounds 89 , 86 , 88 , 85 , and 87 showed significant activity on the three tumor cell lines tested. The results of synthesized compounds showed in Table 18 .
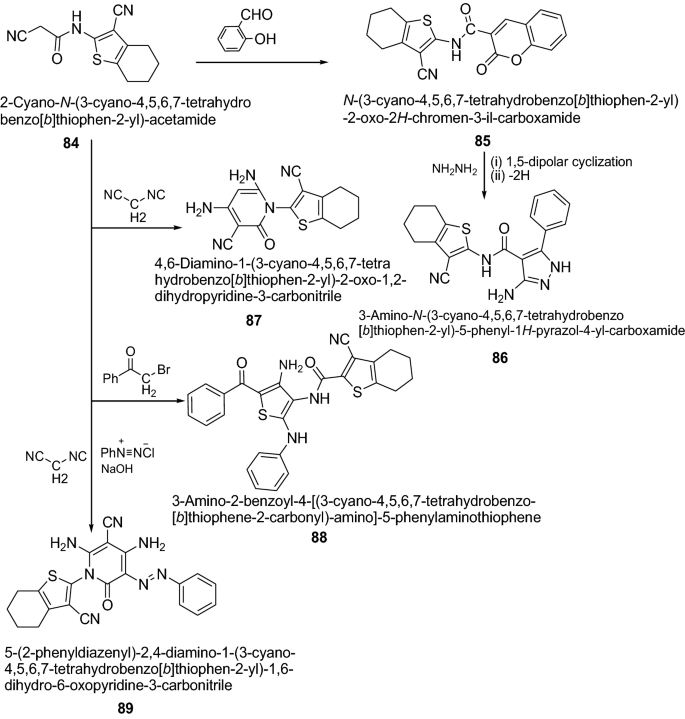
Synthesis ofsubstituted-1-(3-cyano-4,5,6,7-tetrahydrobenzo[ b ]thiophen-2-yl) derivatives
Antioxidant activities
Madhavi et al. [ 34 ] developed a novel class of substituted 2-(2-cyanoacetamido)thiophenes by cyanoacetylation of substituted 2-aminothiophene by using an effective cyanoacetylating agent, 1-cyanoacetyl-3,5-dimethylpyrazole as presented in Scheme 18 . All the synthesized compounds were evaluated for in vitro antioxidant activity by scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH) and nitric oxide free radicals at 100 μM concentration. Among these evaluated compounds, 2-(2-cyanoacetamido)-4,5-dimethylthiophene-3-carboxamide (Compound 92a ) was found to possess highest anti-oxidant activity in both models of free radical scavenging. However in case of assay with nitric oxide free radical scavenging, the highest activity was exhibited by 2-(2-cyanoacetamido)-4,5-dimethylthiophene-3-carboxamide (Compound 92a , 56.9%) and 2-(2-cyanoacetamido)-4,5,6,7-tetrahydrobenzo[ b ]thiophene-3-carboxamide (Compound 92b , 55.5%). The greater activity of these compounds were attributed due to the polar nature of carboxamide or nitrile group at 3rd position on thiophene ring. The results of synthesized compounds presented in Tables 19 and 20 .
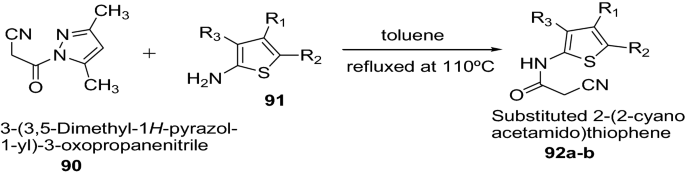
Synthesis of substituted 2-aminothiophene
Anti-inflammatory activity
Bahashwan et al. [ 35 ] synthesized new series of fused triazolo- and tetrazolopyrimidine derivatives (Scheme 19 ) and their anti-inflammatory activity was evaluated. Newly synthesized thienopyrimidine derivatives were screened for anti-inflammatory activity (percent inhibition of edema obtained by the reference drug and tested compounds, respectively) in comparison to that of indomethacin. Among the series, compounds 94 , 95 , 96 , 97 and 98 possess strong anti-inflammatory activity. The high anti-inflammatory activity was mainly due to the presence of electron-donating moieties which increase the pharmacological activity. The order of anti-inflammatory properties with the substitution of electron–donating group in pyrimidine derivatives follows as: hydrazine > methyl > cyanomethyl > tetrazine > amide as exhibited in compounds 94 > 98 > 95 > 96 > 97 , respectively. The results of synthesized compounds presented in Table 21 .
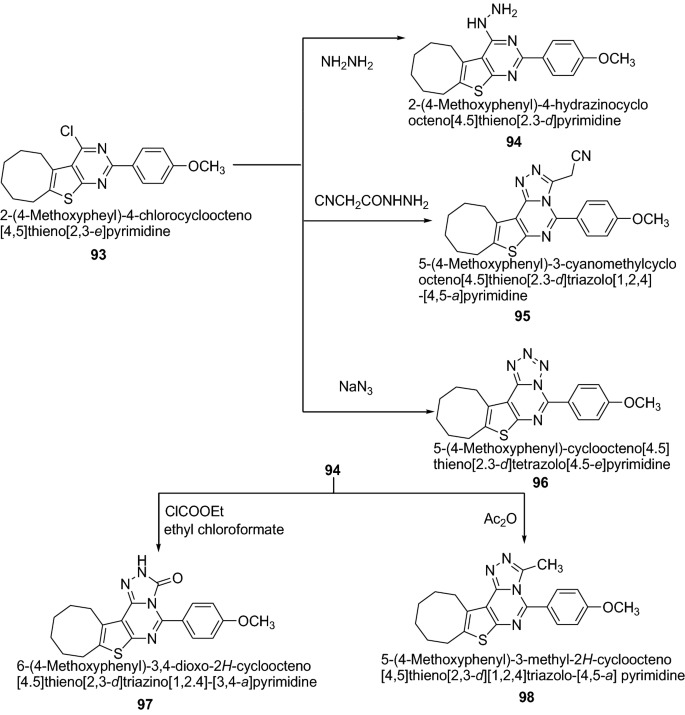
Synthesis of thienotriazolopyrimidine derivatives
Ouf et al. [ 36 ] synthesized hydrazones derivatives which shows significant anti-inflammatory activities as presented in Scheme 20 . The synthesized compounds were screened against the standard drug flurbiprofen. Among the synthesized hydrazones, the substituted 4-methoxy- 100a , 4-chloro- 100b and 4-nitro-derivatives 100c have anti-inflammatory activities higher than that of hydrazone with an unsubstituted benzaldehyde group against the standard drug flurbiprofen. Thus, the lipophilicity plays an important role for the potent anti-inflammatory activity. The results of synthesized compounds presented in Table 22 .
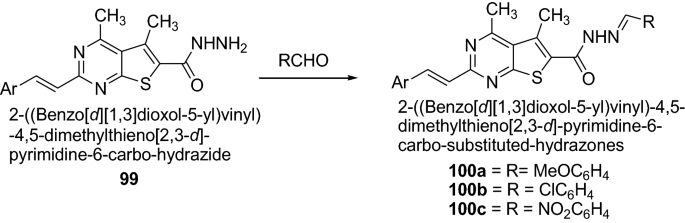
Synthesis of 2-((Benzo[ d ] [ 1 , 3 ] dioxol-5-yl)vinyl)-4,5-dimethylthieno[2,3- d ]-pyrimidine-6-carbohydrazones derivatives
Hafez et al. [ 37 ] synthesized some of the novel benzothino-pyrimidine derivatives (Scheme 21 ) which showed considerable potent anti-inflammatory activity. The anti-inflammatory activity of the newly synthesized compounds were evaluated by applying carrageenan-induced paw edema bioassay in rats using indomethacin as a reference standard. Compounds 105 , 106 , 107 , 108 and 109 caused significant decreases in paw edema after 2, 3, 4 h after drug administration. Thus, it can be concluded that spirobenzothienopyrimidine moiety, phenylpyrazolothinopyrimidine, morphonyl and piperazinylthinopyrimidine ring systems are important for anti-inflammatory activity. The results of synthesized compounds presented in Table 23 .

Synthesis of phenylpyrazolothinopyrimidine, morphonyl and piperazinylthinopyrimidine derivatives
Antiurease activity
Rasool et al. [ 38 ] synthesized variety of novel 5-aryl thiophenes derivatives containing sulphonylacetamide (sulfacetamide) using Scheme 22 . The synthesized compounds were screened for their anti-urease activities by taking thiourea as standard drug. Among all the synthesized derivatives, compound 112 , N -((5′-methyl-[2,2′-bithiophen]-5-yl)sulfonyl)acetamide, showed excellent urease inhibition activity at 40 µg/ml and 80 µg/ml concentrations where the percentage inhibition values were found to be 92.12 ± 0.21 and 94.66 ± 0.11, respectively with an IC 50 value ~ 17.1 ± 0.15 µg/ml. It is further concluded that the urease inhibitory activity of compound might be due to the presence of the electronic and steric effects of functional groups. The results of synthesized compounds are presented in Table 24 .
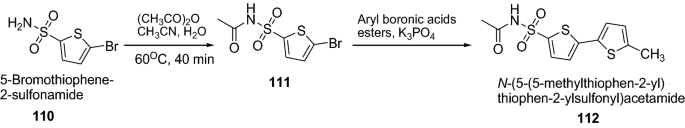
Synthesis of N -(5-(5-methylthiophen-2-yl)thiophen-2 ylsulfonyl)acetamide
Anticonvulsant activity
Dashyan et al. [ 39 ] synthesized 2,4-disubstituted pyr ano[4′′,3′′:4′,5′]pyrido[3′,2′:4,5]thieno[3,2- d ]pyrimidines derivatives by using Scheme 23 . The synthesized compounds were screened for the anticonvulsant activity of by taking the comparator drug, diazepam which was performed using male albino mice weighing 18–24 g (200 animals) and rats (Wistar) weighing 120–140 g (40 animals of both sexes).
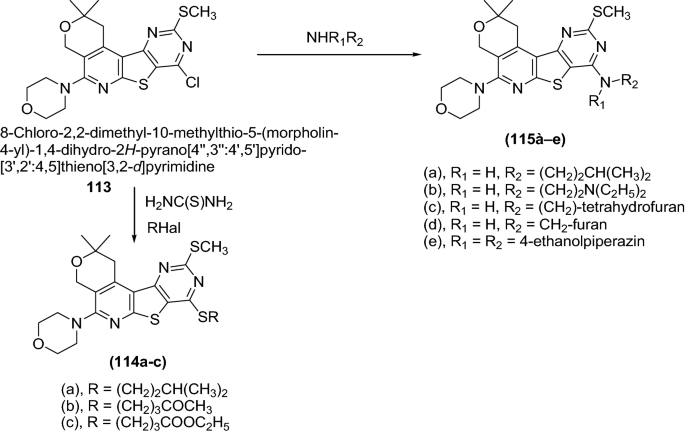
Synthesis of 2,4-Disubstituted pyrano[4′,3′:4,5]pyrido[2,3- b ]thieno[3,2- d ]pyrimidine derivatives
The anticonvulsant activity of the compounds was assessed by the prevention of clonic twitches and the clonic component of convulsions caused by subcutaneous administration of 90 mg/kg metrazol in mice. When studying anticonvulsant activity, it was found that the compounds ( 114a , b , c ) and ( 115a , b , c , d , e ) caused a marked protective anticonvulsive effect, which developed in mice starting with a dose of 25 mg/kg, while statistically calculated dose (ED 50 ) ranged from 23 to 56 mg/kg (Table 25 ).
Antithrombotic activity
Jubair et al. [ 40 ] synthesized novel series of 2-(bromomethyl)-5-aryl-thiophenes derivatives via Suzuki cross-coupling reactions of various aryl boronic acids with 2-bromo-5 (bromomethyl)thiophene as given in Scheme 24 . The synthesized compounds were screened for their antithrombolytic activity. All the Compounds (100 μL) having concentration of 1 mg/ml were added to the micro-centrifuge tubes containing venous blood, and incubated at 37 °C for 45 min. Streptokinase was used as standard clot lysis agent and water as negative control for this assay. Among all the synthesized compounds, 118 showed potent clot lysis (31.5%). However, the results were significant p < 0.05, when compared with streptokinase. Clot lysis activity results are presented in Table 26 .
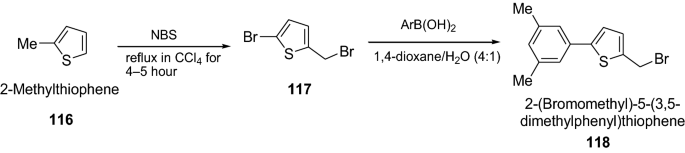
Synthesis of 2-(Bromomethyl)-5-(3,5-dimethylphenyl)thiophene
The analytical and other informational data, available in literature so far, have reveals that thiophene and its derivatives represent an important class of compounds in the medicinal field with various therapeutic potentials, i.e., antimalarial, antimicrobial, antimycobacterial, antidepressant, anticonvulsant, antiviral, anticancer, antihypertensive, anti-inflammatory and antioxidant. Appraisal of literature reports reveals that thiophene moiety have hiked a great deal of interests of medicinal chemist and biochemist to plan, organize and implement new approaches towards discovery of novel drugs.
This particular review article, established the fact that thiophene derivatives could be a rich source of potential entities in search of new generation of biologically active compounds and be worthwhile to explore the possibility in this area by fusing differently substituted moieties which may result in better pharmacological activities. Thus the quest to explore many more modifications on thiophene moiety needs to be continued.
Patel AA, Mehta AG (2010) Synthesis of novel heterocyclic compounds and their biological evaluation. Der Pharm Chem 2(1):215–223
CAS Google Scholar
Mishra R, Jha KK, Kumar S, Tomer I (2011) Synthesis, properties and biological activity of thiophene: a review. Der Pharm Chem 3(4):38–54
Chaudhary A, Jha KK, Kumar S (2012) Biological diversity of thiophene: a review. J Adv Sci Res 3(3):03–10
Mishra R, Sharma PK (2015) A review on synthesis and medicinal importance of thiophene. Int J Eng Al Sci 1(1):46–59
Google Scholar
Chambhare RV, Khadse BG, Bobde AS (2003) Synthesis and preliminary evaluation of some N -[5-(2-furanyl)-2-methyl-4-oxo-4 H -thieno[2,3- d ]pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3 H -thieno[2,3- d ]pyrimidin-4-ones as antimicrobial agents. Euro J Med Chem 38(1):89–100
Article CAS Google Scholar
Tehranchian S, Akbarzadeh T, Fazeli MR, Jamalifar H, Shafiee A (2005) Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydro-benzo[ c ]thiophen-4(5 H )ones. Bioorg Med Chem Lett 15:1023–1025
Article CAS PubMed Google Scholar
Pillai AD, Rathod PD, Xavier FP, Pad H, Sudarsanam V, Vasu KK (2005) Tetra substituted thiophenes as anti-inflammatory agents: exploitation of analogue-based drug design. Bioorg Med Chem 13:6685–6692
Russell RK, Press JB, Rampulla RA, McNally JJ, Falotico R, Keiser JA, Bright DA, Tobia A (1988) Thiophene systems: thienopyrimidinedione derivatives as potential antihypertensive agents. J Med Chem 31:1786–1793
Chen Z, Ku TC, Seley KL (2015) Thiophene-expanded guanosine analogues of gemcitabine. Bioorg Med Chem Lett 25:4274–4276
Article CAS PubMed PubMed Central Google Scholar
Benabdellah M, Aouniti A, Dafali A, Hammouti B, Benkaddour M, Yahyi A, Ettouhami A (2006) Investigation of the inhibitive effect of triphenyltin-2-thiophene carboxylate on corrosion of steel in 2 M H3PO4 solutions. Appl Surf Sci 252:8341–8347
Kim C, Choi KS, Oh JH, Hong HJ, Han SH, Kim SY (2015) The effects of octylthiophene ratio on the performance of thiophene based polymer light-emitting diodes. Sci Adv Mater 7:2401–2409
Mishra R, Jha KK, Kumar S, Tomer I (2012) Thiophene: the molecule of diverse medicinal importance. J Pharm Res 5(1):560–566
Priyanka SNK, Jha KK (2010) Benzothiazole: the molecule of diverse biological activities. Inter J Curr Pharm Res 2(2):01–06
Mehta A, Bhatt R, Sharma S, Patidar AK, Rathore KK, Senwar RC (2015) Synthesis, characterization and antimicrobial evaluation of some tetrahydroquinazoline derivatives of benzo[ b ]thiophene. Int J Pharm Sci Drug Res 7(5):417–420
Mazimba O (2015) Antimicrobial activities of heterocycles derived from thienylchalcones. J King Saud Univ Sci 27:42–48
Article Google Scholar
Prasad KC, Angothu BN, Latha TM, Nagulu M (2017) Synthesis of some novel 2-aminothiophene derivatives and evaluation for their antimicrobial activity. Int J Pharm Bio Sci 7(1):01–07
Lakshmi N, Haritha V, Sreeram V, Rajalakshmi D, Sindhura N, Visagaperumal D (2009) Synthesis and their possible biological activities of few formazans of 3-amino-2-sulphanyl-2,3,4,5,6,7,8-hexahydro(1)benzothieno(2,3- d )pyrimidin-4(1 H )-one. Rayasan J Chem 2(1):71–74
Havaldar FH, Bhise S, Burudkar S (2004) A facile synthesis of 10-methoxy-4,8-dinitro-6 H -benzothieno[2,3- c ]chromen-6-one. J Serb Chem Soc 69(7):527–532
Ahmed MM, Matough FS, Farhat MF (2008) Synthesis and biological evaluation of some new thienopyridine and thienopyrimidine derivatives. Jord J Chem 3(3):223–232
Bhuiyan MH, Rahman M, Hossain K, Rahim A (2006) Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives. Acta Pharm 56:441–450
CAS PubMed Google Scholar
Khazi IA, Kumar N, Shetty S, Lamani RS (2009) Synthesis and antimicrobial activity of some novel thienopyrimidines and triazolothienopyrimidines. J Chem Sci 121(3):301–307
Tombary AA, Soliman R, Habib NS (2009) Synthesis of tetrahydrobenzothieno[2,3- d ]pyrimidine and tetrahydrobenzothieno[3,2- e ]-[1,2,4]triazolo[4,3- c ]pyrimidine derivatives as potential antimicrobial agents. Sci Pharm 77:755–773
Adiwish WM, Yaacob WA, Adan D, Nazlina I (2012) Synthesis and antibacterial activity of thiophenes. Int J Adv Sci Eng Inf Tech 2(4):27–30
Reheim AM, Baker SM (2017) Synthesis, characterization and in vitro antimicrobial activity of novel fused pyrazolo[3,4- c ]pyridazine, pyrazolo[3,4- d ] pyrimidine, thieno[3,2- c ]pyrazole and pyrazolo[3′,4′:4,5]thieno[2,3- d ]pyrimidine derivatives. Chem Cent J 11:112–124
Article PubMed PubMed Central CAS Google Scholar
Bindu PJ, Mahadevan KM, Naik TR (2012) An efficient one-pot synthesis and photoinduced DNA cleavage studies of 2-chloro-3-(5-aryl-4,5-dihydroisoxazol-3-yl)quinolines. Bioorg Med Chem Lett 22(19):6095–6098. https://doi.org/10.1016/j.bmcl.2012.08.034
Dosari MS, Ghorab MM, Alsaid MS, Nissan YM, Ahmed AM (2013) Synthesis and anticancer activity of some novel trifluoromethylquinolines carrying a biologically active benzenesulfonamide moiety. Eur J Med Chem 69:373–383
Sankaran M, Kumarasamy C, Chokkalingam U, Mohan PS (2010) Synthesis, antioxidant and toxicological study of novel pyrimidoquinoline derivatives from 4-hydroxy-3-acylquinolin-2-one. Bioorg Med Chem Lett 20:7147–7151
Ghorab MM, Bashandy MS, Alsaid MS (2014) Novel thiophene derivatives with sulfonamide, isoxazole, benzothiazole, quinoline and anthracene moieties as potential anticancer agents. Acta Pharm 64:419–431
Gunda SR, Lingala S, Allenki V (2017) Synthesis and anticancer activity of some novel 3-[(2-substituted-6,7,8,9-tetrahydro-5 H cyclohepta[ b ]thieno[2,3- d ]pyrimidin-4-yl)amino]propan-1-ol derivatives. Eur J Pharm Med Res 4(6):481–484
Mohareb RM, Abdallah AE, Helal MH, Shaloof SM (2016) Synthesis and structure elucidation of some novel thiophene and benzothiophene derivatives as cytotoxic agents. Acta Pharm 66:53–68
Sharkawy KA, Sehrawi HM, Ibrahim RA (2012) The reaction of 2-amino-4,5,6,7-tetrahydrobenzo[ b ]thiophenes with benzoyl-isothiocyanate: synthesis of annulated thiophene derivatives and their antitumor evaluations. Inter J Org Chem 2:126–134
Seley KL, Januszczyk P, Hagos A, Zhang L (2000) Synthesis and antitumor activity of thieno-separated tricyclic purines. J Med Chem 43:4877–4883
Mohareb RM, Shams HZ, Helal MH, Mahmoud AE (2011) Novel synthesis and antitumor evaluation of polyfunctionally substituted heterocyclic compounds derived from 2-cyano- N -(3-cyano-4,5,6,7-tetrahydrobenzo[ b ]thiophen-2-yl)-acetamide. Molecules 16:52–73
Madhavi K, Soumya KR, Subhashini C (2017) Cyanoacetylation of substituted 2-aminothiophenes and evaluation for antioxidant and antibacterial activities. Res J Pharm Biol Chem Sci 8(2):387–394
Bahashwan SA, Fayed AA, Amr AG, Flefel EM, Kalmouch A (2013) Synthesis and pharmacological activities of some new triazoloand tetrazolopyrimidine derivatives. Molecules 18:15051–15063
Ouf NH, Amr AG (2008) Synthesis and anti-inflammatory activity of some pyrimidines and thienopyrimidines using 1-(2-Benzo[ d ][1,3]dioxol-5-yl)vinyl)-4-mercapto-6-methylpyrimidine-5-yl)ethan-2-one as a starting material. Monatsh Chem 139:579–585
Hafez HN, Duaij OK, Gazzar AB (2013) Design, synthesis and pharmacological evaluation of new nonsteroidal anti-inflammatory derived from 3-aminobenzothieno[2,3- d ]pyrimidines. Inter J Org Chem 3:110–118
Rasool N, Noreen M (2015) Synthesis, density functional theory (DFT), urease inhibition and antimicrobial activities of 5-aryl thiophenes bearing sulphonylacetamide moieties. Molecules 20:19914–19928
Dashyan Paronikyan EG, Noravyan AS (2015) Synthesis and neurotropic activity of 2,4-disubstituted pyrano[4′,3′:4,5]pyrido[2,3- b ]thieno[3,2- d ]pyrimidines. Russian J Bioorg Chem 41(6):663–669
Zubair M, Rizwan K (2014) Regioselective synthesis of 2-(bromomethyl)-5-aryl-thiophene derivatives via palladium (0) catalyzed suzuki cross-coupling reactions: as antithrombotic and haemolytically active molecules. Chem Cent J 8(74):1–8
Download references
Authors’ contributions
PKV designed and finalized the scheme; RS performed review work and wrote the paper. Both authors read and approved the final manuscript.
Acknowledgements
Thanks to Head, Department of Pharmaceutical Sciences, MD. University, Rohtak for kind support for providing internet facilities etc.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and affiliations.
Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, Haryana, 124001, India
Rashmi Shah & Prabhakar Kumar Verma
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Prabhakar Kumar Verma .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Shah, R., Verma, P.K. Therapeutic importance of synthetic thiophene. Chemistry Central Journal 12 , 137 (2018). https://doi.org/10.1186/s13065-018-0511-5
Download citation
Received : 04 March 2018
Accepted : 04 December 2018
Published : 19 December 2018
DOI : https://doi.org/10.1186/s13065-018-0511-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heterocyclic compounds
- Combinatorial library
- Antimicrobial
BMC Chemistry
ISSN: 2661-801X
- General enquiries: [email protected]

Submission guidelines
Types of papers, 1. general information, manuscript submission, artwork and illustrations guidelines, supplementary information (si), after acceptance.
Open Choice
Research Data Policy and Data Availability Statements
Scientific style, additional information for scientific style, ethical responsibilities of authors, competing interests, research involving human participants, their data or biological material, research involving animals, their data or biological material, informed consent, authorship principles, research involving human embryos, gametes and stem cells, utilization of plants, algae, fungi, editorial procedure, editing services, open access publishing.
- Mistakes to avoid during manuscript preparation
Instructions for Authors
- Brief Reports
- Original Research Articles
Detailed Instructions for Authors (Download pdf, 1 MB)
Medicinal Chemistry Research is a journal for the prompt disclosure of novel experimental achievements in many facets of drug design, drug discovery, and the elucidation of mechanisms of action of biologically active compounds. Articles are sought which emphasize research in chemical biological relationships, especially with respect to: structure-activity relationships, investigations of biochemical and pharmacological targets of drug action, and correlations of structures with the mode of action of biologically active compounds. Studies will be welcomed that increase our understanding of biochemical interactions between drug molecules, ions, free radicals, and sterically important sections of macromolecular targets. The Journal is also dedicated to medicinal plants and to bioactive natural products of plant, fungal, mammalian, and aquatic origin. The Journal publishes original contributions in the following major areas:
- Design, synthesis, and structure-activity relationships of bioactive compounds
- Docking, molecular modeling, and computational studies of bioactive interactions
- Characterization of active ingredients of medicinal plants and identification of bioactivity in plant extracts
- Identification of targets and mechanism of activity of bioactive compounds
- Chemistry and biochemistry of bioactive natural products of plant origin
- Critical reviews of the historical, clinical, and legal status of medicinal agents, and accounts on topical issues.
Contributions reporting the following are not normally considered for publication:
- Biological activity on crude extracts that have not been characterized by analysis of their secondary metabolites (HPLC, 1H and 13C NMR including 2D NMR).
- Unexceptional and predictable bioactivity (e.g. antioxidant properties of phenolic or antibacterial activity of essential oils or antioxidant properties of metals such as iron, copper, etc.).
- Uncritical ethnopharmacological investigations, where a list of plants and their use are simply reported.
- Synthetic work in which the spectroscopic characterization is not complete (e.g., 1H and 13C NMR, HRMS, CHN, UV, IR, etc.).
- Computational work that simply discusses the docking, molecular modeling, QSAR, SAR, and computational studies of bioactive interactions without validation of the method (with experimental data).
- Biological activity that is low and insufficient to generate meaningful structure activity relationship.
Violation of any of the following rules will result in an immediate rejection:
RULE 1: The manuscript does not fall into any of the areas of interest of the Journal.
RULE 2: The manuscript is too preliminary (e.g., data without comparison to a reference, or without a positive control).
RULE 3: The botanical source is not clearly identified, authenticated, or documented (voucher specimen).
RULE 4: The manuscript is too focused on a non-chemical subject (e.g., pharmacology, analytical studies of active ingredients, analytical studies of drug concentrations (ADME is suitable), etc.
RULE 5: Manuscripts that simply discuss antioxidant properties of phenols or other compounds known to possess antioxidant effects.
RULE 6: Computer QSAR/modeling manuscripts that lack experimental biological validation of the proposed model(s).
RULE 7: The manuscript does not follow the formatting provided in this document.
RULE 8: The manuscripts contains poor English or difficult to read language.
2. General Considerations and types of manuscripts
Authors are strongly encouraged to provide their manuscript in an electronic format. The text must be in a single-column format and lines with double space. Use plain font 12 point Times New Roman and symbols (use internationally accepted signs and symbols for units, SI units). Use the automatic page numbering function to number all the pages. Ensure that all special characters are presented in the body of the text and do not use graphics. Abbreviations, except for very common ones, must be defined the first time they are used and a list supplied with the manuscript.
Using clear and concise English will help the editors and the reviewers concentrate on the scientific merit of the manuscript and thus smooth the peer review process. We reject manuscripts with good science that are poorly written.
The text of a research manuscript should be divided into the following sections: Introduction, Results and Discussion, Conclusions, Materials and Methods/Experimental, Acknowledgements (Funding), Conflict of Interest, and References as appropriate for the specific manuscript type. Tables, figures, and schemes should be embedded in the text. Do not upload tables, figures, and schemes that are to be published in the manuscript into the electronic supplementary material. Authors are encouraged to provide supplementary material to keep the manuscript to a reasonable length appropriate for the specific manuscript type.
Types of manuscript accepted include comments, reviews, brief reports, and original research articles.
2.1. Guidelines for comments
Comments are normally by invitation only. Unsolicited comment articles are rarely considered, but if you wish to enquire further about the suitability of your article, you can email Dr. Hans Detlef Klueber ([email protected]).
Comments are short focused articles or viewpoints that are usually invited by the journal. These articles are generally peer-reviewed in consultation with the journal's Editorial Board. A comment article can be editorial in nature discussing new topics or new technologies in medicinal chemistry research and can also be used to highlight one or more exciting research articles recently published in Med. Chem. Res.
Comments should be less than 3,000 words with less than 15 references, preferably <5 references. No abstract or graphical abstract is needed. Supplementary materials are allowed.
2.2. Guidelines for review articles
Review articles are peer-reviewed articles covering important topic areas that are of significant interest to the broad community of scientists working in drug design, discovery, and development. They are typically 10-20 pages in length. We also welcome shorter, mini-review style articles under this article type that cover new drugs recently developed and tested in clinical trials and/or received regulatory approval.
Review articles do not need to be comprehensive and should focus on important drug targets, mechanism of action, and/or important structure-activity relationship findings and key physicochemical/pharmaceutical/DMPK properties that made the molecule into a drug. Authors should summarize important findings rather than reiterating details published in the primary literature. Whenever possible, authors should make their own summary figures instead of reusing multiple figures from the primary literature. If reusing or simply adapting figures already published, authors need to obtain permission from the copy right holders and reference accordingly.
Authors should include balanced coverage of the field reviewed and not just their own research. The authors are encouraged to incorporate their own perspectives and do their own analysis of data published. In the final section, the authors should discuss future directions and trends where appropriate.
2.3. Guidelines for brief reports
Brief reports are peer-reviewed short papers that present original and significant results in drug design, discovery, and development for rapid dissemination or short notes that present technical developments and innovations in the use of new technologies such as artificial intelligence, machine learning, high throughput experimentation, flow chemistry, and software to advance drug discovery and development.
Brief reports are limited to 6 pages in length. The paper should contain an abstract, graphical abstract, main body and references, and contain no more than 6 figures, schemes, or tables, combined. The abstract is limited to 150 words. The paper may not need an experimental section but details about compound characterization and purity confirmation can be placed in a supporting document.
2.4. Guidelines for original research articles
Original research articles are peer-reviewed full papers that present original and significant results in drug design, discovery, and development with an experimental section. There are no page limits, although most original research articles would have around 8-15 journal pages.
The text of an original research article should be divided into the following sections: Introduction, Results and Discussion, Conclusions, Materials and Methods/Experimental, Acknowledgements (Funding), Conflict of Interest, and References. Tables, figures, and schemes should be embedded in the text or be included right after the references on separate pages (one each per page). The authors should limit the number of schemes/figures/tables to a total of 10 or less in the manuscript.
2.5. Manuscript requirements at a glance
| Article type | Brief description | Word count/ # print pages/ # illustrations | Abstract length | Graphical abstract | # Keywords | # references |
|---|---|---|---|---|---|---|
| 1. Comments |
With or without figures +schemes +tables | NOT NEEDED | NOT NEEDED | Optional | ≤15, preferably | |
| 2. Reviews | ~5,000 -10,000 words ~10-20 print pages ~10 figures +schemes +tables, combined | ≤250 words | YES | 4-6 | ~100-200 | |
| 3. Brief Reports |
≤6 figures +schemes +tables, combined | ≤150 words | YES | 4-6 | ≤30 | |
| 4. Original Research Articles | ~5,000 -10,000 words ~10-20 print pages ~10 figures +schemes +tables, combined | ≤250 words | YES | 4-6 | ~50 |
Graphical Abstract. The author(s) are required to provide a graphical abstract that is a single schematic image which visually represents the main findings of the article, allowing readers to easily capture the content of the article at a single glance. The graphical abstract must meet the same quality and permissions standards as any other figure in the article. A caption is not needed, while compound numbers can be given in the graphical abstract. The use of color is encouraged and there is no charge for the graphical abstract. The graphical abstract should be legible at a size of 5 × 3 inches (130 × 75 mm, w × h)) using a regular screen resolution of 96 dpi. Graphical abstracts can be selected as the front cover image.
Cover Image. Authors are encouraged to submit a high resolution image of their graphical abstract as a separate file for consideration as a cover image at the revision/proofing stage. The image file should be created at publication size: 8 × 4.8 in (200 × 120 mm, w × h) at a resolution of at least 300 dpi in uncompressed JPEG, TIFF, PNG, PSD, AI, EPS, or PDF format.
Immediately after the abstract paragraph/graphical abstract, 4 to 6 keywords, should be provided for indexing purposes under the heading Keywords.
Submission of a manuscript implies: that the work described has not been published before; that it is not under consideration for publication anywhere else; that its publication has been approved by all co-authors, if any, as well as by the responsible authorities – tacitly or explicitly – at the institute where the work has been carried out. The publisher will not be held legally responsible should there be any claims for compensation.
Permissions
Authors wishing to include figures, tables, or text passages that have already been published elsewhere are required to obtain permission from the copyright owner(s) for both the print and online format and to include evidence that such permission has been granted when submitting their papers. Any material received without such evidence will be assumed to originate from the authors.
Online Submission
Please follow the hyperlink “Submit manuscript” and upload all of your manuscript files following the instructions given on the screen.
Source Files
Please ensure you provide all relevant editable source files at every submission and revision. Failing to submit a complete set of editable source files will result in your article not being considered for review. For your manuscript text please always submit in common word processing formats such as .docx or LaTeX.
Submitting Declarations
Please note that Author Contribution information and Competing Interest information must be provided at submission via the submission interface. Only the information submitted via the interface will be used in the final published version. Please make sure that if you are an editorial board member and also a listed author that you also declare this information in the Competing Interest section of the interface.
Please see the relevant sections in the submission guidelines for further information on these statements as well as possible other mandatory statements.
Please make sure your title page contains the following information
The title should be concise and informative.
Author information
- The name(s) of the author(s)
- A concise and informative title
- The affiliation(s) of the author(s), i.e. institution, (department), city, (state), country
- A clear indication and an active e-mail address of the corresponding author
- If available, the 16-digit ORCID of the author(s)
If address information is provided with the affiliation(s) it will also be published.
For authors that are (temporarily) unaffiliated we will only capture their city and country of residence, not their e-mail address unless specifically requested.
Large Language Models (LLMs), such as ChatGPT , do not currently satisfy our authorship criteria . Notably an attribution of authorship carries with it accountability for the work, which cannot be effectively applied to LLMs. Use of an LLM should be properly documented in the Methods section (and if a Methods section is not available, in a suitable alternative part) of the manuscript. The use of an LLM (or other AI-tool) for "AI assisted copy editing" purposes does not need to be declared. In this context, we define the term "AI assisted copy editing" as AI-assisted improvements to human-generated texts for readability and style, and to ensure that the texts are free of errors in grammar, spelling, punctuation and tone. These AI-assisted improvements may include wording and formatting changes to the texts, but do not include generative editorial work and autonomous content creation. In all cases, there must be human accountability for the final version of the text and agreement from the authors that the edits reflect their original work.
Please provide an abstract of 150 to 250 words. The abstract should not contain any undefined abbreviations or unspecified references.
For life science journals only (when applicable)
- Trial registration number and date of registration for prospectively registered trials
- Trial registration number and date of registration, followed by “retrospectively registered”, for retrospectively registered trials
Please provide 4 to 6 keywords which can be used for indexing purposes.
Statements and Declarations
- Competing Interests: Authors are required to disclose financial or non-financial interests that are directly or indirectly related to the work submitted for publication. Please refer to "Competing Interests and Funding" below for more information on how to complete this section.
Please see the relevant sections in the submission guidelines for further information as well as various examples of wording. Please revise/customize the sample statements according to your own needs.
Text Formatting
Manuscripts should be submitted in Word.
- Use a normal, plain font (e.g., 10-point Times Roman) for text.
- Use italics for emphasis.
- Use the automatic page numbering function to number the pages.
- Do not use field functions.
- Use tab stops or other commands for indents, not the space bar.
- Use the table function, not spreadsheets, to make tables.
- Use the equation editor or MathType for equations.
- Save your file in docx format (Word 2007 or higher) or doc format (older Word versions).
Manuscripts with mathematical content can also be submitted in LaTeX. We recommend using Springer Nature’s LaTeX template .
Please use no more than three levels of displayed headings.
Abbreviations
Abbreviations should be defined at first mention and used consistently thereafter.
Footnotes can be used to give additional information, which may include the citation of a reference included in the reference list. They should not consist solely of a reference citation, and they should never include the bibliographic details of a reference. They should also not contain any figures or tables.
Footnotes to the text are numbered consecutively; those to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data). Footnotes to the title or the authors of the article are not given reference symbols.
Always use footnotes instead of endnotes.
Acknowledgments
Acknowledgments of people, grants, funds, etc. should be placed in a separate section after Experimental. The names of funding organizations should be written in full.
Reference citations in the text should be identified by numbers in square brackets. Some examples:
1. Negotiation research spans many disciplines [3].
2. This result was later contradicted by Becker and Seligman [5].
3. This effect has been widely studied [1-3, 7].
Reference list
The list of references should only include works that are cited in the text and that have been published or accepted for publication. Personal communications and unpublished works should only be mentioned in the text.
The entries in the list should be numbered consecutively.
If available, please always include DOIs as full DOI links in your reference list (e.g. “https://doi.org/abc”).
Smith JJ. The world of science. Am J Sci. 1999;36:234–5.
Slifka MK, Whitton JL. Clinical implications of dysregulated cytokine production. J Mol Med. 2000; https://doi.org/10.1007/s001090000086
Blenkinsopp A, Paxton P. Symptoms in the pharmacy: a guide to the management of common illness. 3rd ed. Oxford: Blackwell Science; 1998.
Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. In: Bourne GH, Danielli JF, Jeon KW, editors. International review of cytology. London: Academic; 1980. pp. 251–306.
Doe J. Title of subordinate document. In: The dictionary of substances and their effects. Royal Society of Chemistry. 1999. http://www.rsc.org/dose/title of subordinate document. Accessed 15 Jan 1999.
Always use the standard abbreviation of a journal’s name according to the ISSN List of Title Word Abbreviations, see
ISSN.org LTWA
If you are unsure, please use the full journal title.
For authors using EndNote, Springer provides an output style that supports the formatting of in-text citations and reference list. Please click link below to download the EndNote style file.
SpringerVancouverNumber.ens
- All tables are to be numbered using Arabic numerals.
- Tables should always be cited in text in consecutive numerical order.
- For each table, please supply a table caption (title) explaining the components of the table.
- Identify any previously published material by giving the original source in the form of a reference at the end of the table caption.
- Footnotes to tables should be indicated by superscript lower-case letters (or asterisks for significance values and other statistical data) and included beneath the table body.
Electronic Figure Submission
- Supply all figures electronically.
- Indicate what graphics program was used to create the artwork.
- For vector graphics, the preferred format is EPS; for halftones, please use TIFF format. MSOffice files are also acceptable.
- Vector graphics containing fonts must have the fonts embedded in the files.
- Name your figure files with "Fig" and the figure number, e.g., Fig1.eps.
- Definition: Black and white graphic with no shading.
- Do not use faint lines and/or lettering and check that all lines and lettering within the figures are legible at final size.
- All lines should be at least 0.1 mm (0.3 pt) wide.
- Scanned line drawings and line drawings in bitmap format should have a minimum resolution of 1200 dpi.
Halftone Art
- Definition: Photographs, drawings, or paintings with fine shading, etc.
- If any magnification is used in the photographs, indicate this by using scale bars within the figures themselves.
- Halftones should have a minimum resolution of 300 dpi.
Combination Art
- Definition: a combination of halftone and line art, e.g., halftones containing line drawing, extensive lettering, color diagrams, etc.
- Combination artwork should have a minimum resolution of 600 dpi.
- Color art is free of charge for online publication.
- If black and white will be shown in the print version, make sure that the main information will still be visible. Many colors are not distinguishable from one another when converted to black and white. A simple way to check this is to make a xerographic copy to see if the necessary distinctions between the different colors are still apparent.
- If the figures will be printed in black and white, do not refer to color in the captions.
- Color illustrations should be submitted as RGB (8 bits per channel).

Figure Lettering
- To add lettering, it is best to use Helvetica or Arial (sans serif fonts).
- Keep lettering consistently sized throughout your final-sized artwork, usually about 2–3 mm (8–12 pt).
- Variance of type size within an illustration should be minimal, e.g., do not use 8-pt type on an axis and 20-pt type for the axis label.
- Avoid effects such as shading, outline letters, etc.
- Do not include titles or captions within your illustrations.
Figure Numbering
- All figures are to be numbered using Arabic numerals.
- Figures should always be cited in text in consecutive numerical order.
- Figure parts should be denoted by lowercase letters (a, b, c, etc.).
- If an appendix appears in your article and it contains one or more figures, continue the consecutive numbering of the main text. Do not number the appendix figures,"A1, A2, A3, etc." Figures in online appendices [Supplementary Information (SI)] should, however, be numbered separately.
Figure Captions
- Each figure should have a concise caption describing accurately what the figure depicts. Include the captions in the text file of the manuscript, not in the figure file.
- Figure captions begin with the term Fig. in bold type, followed by the figure number, also in bold type.
- No punctuation is to be included after the number, nor is any punctuation to be placed at the end of the caption.
- Identify all elements found in the figure in the figure caption; and use boxes, circles, etc., as coordinate points in graphs.
- Identify previously published material by giving the original source in the form of a reference citation at the end of the figure caption.
Figure Placement and Size
- Figures should be submitted separately from the text, if possible.
- When preparing your figures, size figures to fit in the column width.
- For large-sized journals the figures should be 84 mm (for double-column text areas), or 174 mm (for single-column text areas) wide and not higher than 234 mm.
- For small-sized journals, the figures should be 119 mm wide and not higher than 195 mm.
If you include figures that have already been published elsewhere, you must obtain permission from the copyright owner(s) for both the print and online format. Please be aware that some publishers do not grant electronic rights for free and that Springer will not be able to refund any costs that may have occurred to receive these permissions. In such cases, material from other sources should be used.
Accessibility
In order to give people of all abilities and disabilities access to the content of your figures, please make sure that
- All figures have descriptive captions (blind users could then use a text-to-speech software or a text-to-Braille hardware)
- Patterns are used instead of or in addition to colors for conveying information (colorblind users would then be able to distinguish the visual elements)
- Any figure lettering has a contrast ratio of at least 4.5:1
Generative AI Images
Please check Springer’s policy on generative AI images and make sure your work adheres to the principles described therein.
Springer accepts electronic multimedia files (animations, movies, audio, etc.) and other supplementary files to be published online along with an article or a book chapter. This feature can add dimension to the author's article, as certain information cannot be printed or is more convenient in electronic form.
Before submitting research datasets as Supplementary Information, authors should read the journal’s Research data policy. We encourage research data to be archived in data repositories wherever possible.
- Supply all supplementary material in standard file formats.
- Please include in each file the following information: article title, journal name, author names; affiliation and e-mail address of the corresponding author.
- To accommodate user downloads, please keep in mind that larger-sized files may require very long download times and that some users may experience other problems during downloading.
- High resolution (streamable quality) videos can be submitted up to a maximum of 25GB; low resolution videos should not be larger than 5GB.
Audio, Video, and Animations
- Aspect ratio: 16:9 or 4:3
- Maximum file size: 25 GB for high resolution files; 5 GB for low resolution files
- Minimum video duration: 1 sec
- Supported file formats: avi, wmv, mp4, mov, m2p, mp2, mpg, mpeg, flv, mxf, mts, m4v, 3gp
Text and Presentations
- Submit your material in PDF format; .doc or .ppt files are not suitable for long-term viability.
- A collection of figures may also be combined in a PDF file.
Spreadsheets
- Spreadsheets should be submitted as .csv or .xlsx files (MS Excel).
Specialized Formats
- Specialized formats such as .pdb (chemical), .wrl (VRML), .nb (Mathematica notebook), and .tex can also be supplied.
Collecting Multiple Files
- It is possible to collect multiple files in a .zip or .gz file.
- If supplying any supplementary material, the text must make specific mention of the material as a citation, similar to that of figures and tables.
- Refer to the supplementary files as “Online Resource”, e.g., "... as shown in the animation (Online Resource 3)", “... additional data are given in Online Resource 4”.
- Name the files consecutively, e.g. “ESM_3.mpg”, “ESM_4.pdf”.
- For each supplementary material, please supply a concise caption describing the content of the file.
Processing of supplementary files
- Supplementary Information (SI) will be published as received from the author without any conversion, editing, or reformatting.
In order to give people of all abilities and disabilities access to the content of your supplementary files, please make sure that
- The manuscript contains a descriptive caption for each supplementary material
- Video files do not contain anything that flashes more than three times per second (so that users prone to seizures caused by such effects are not put at risk)
Upon acceptance, your article will be exported to Production to undergo typesetting. Shortly after this you will receive two e-mails. One contains a request to confirm your affiliation, choose the publishing model for your article, as well as to arrange rights and payment of any associated publication cost. A second e-mail containing a link to your article’s proofs will be sent once typesetting is completed.
Article publishing agreement
Depending on the ownership of the journal and its policies, you will either grant the Publisher an exclusive licence to publish the article or will be asked to transfer copyright of the article to the Publisher.
Offprints can be ordered by the corresponding author.
Color illustrations
Online publication of color illustrations is free of charge. For color in the print version, authors will be expected to make a contribution towards the extra costs.
Proof reading
The purpose of the proof is to check for typesetting or conversion errors and the completeness and accuracy of the text, tables and figures. Substantial changes in content, e.g., new results, corrected values, title and authorship, are not allowed without the approval of the Editor.
After online publication, further changes can only be made in the form of an Erratum, which will be hyperlinked to the article.
Online First
The article will be published online after receipt of the corrected proofs. This is the official first publication citable with the DOI. After release of the printed version, the paper can also be cited by issue and page numbers.
Open Choice allows you to publish open access in more than 1850 Springer Nature journals, making your research more visible and accessible immediately on publication.
Article processing charges (APCs) vary by journal – view the full list
- Increased researcher engagement: Open Choice enables access by anyone with an internet connection, immediately on publication.
It is easy to find funding to support open access – please see our funding and support pages for more information.
*) Within the first three years of publication. Springer Nature hybrid journal OA impact analysis, 2018.
Funding and Support pages
Open Choice articles do not require transfer of copyright as the copyright remains with the author. In opting for open access, the author(s) agree to publish the article under a Creative Commons license. Details of the OA licences offered to authors can be found on the individual journal website, in the journal's How to publish with us guide.
This journal follows Springer Nature research data policy . Sharing of all relevant research data is strongly encouraged and authors must add a Data Availability Statement to original research articles.
Research data includes a wide range of types, including spreadsheets, images, textual extracts, archival documents, video or audio, interview notes or any specialist formats generated during research.
Data availability statements
All original research must include a data availability statement. This statement should explain how to access data supporting the results and analysis in the article, including links/citations to publicly archived datasets analysed or generated during the study. Please see our full policy here .
If it is not possible to share research data publicly, for instance when individual privacy could be compromised, this statement should describe how data can be accessed and any conditions for reuse. Participant consent should be obtained and documented prior to data collection. See our guidance on sensitive data for more information.
When creating a data availability statement, authors are encouraged to consider the minimal dataset that would be necessary to interpret, replicate and build upon the findings reported in the article.
Further guidance on writing a data availability statement, including examples, is available at:
Data repositories
Authors are strongly encouraged to deposit their supporting data in a publicly available repository. Sharing your data in a repository promotes the integrity, discovery and reuse of your research, making it easier for the research community to build on and credit your work.
See our data repository guidance for information on finding a suitable repository.
We recommend the use of discipline-specific repositories where available. For a number of data types, submission to specific public repositories is mandatory.
See our list of mandated data types .
The journal encourages making research data available under open licences that permit reuse. The journal does not enforce use of particular licences in third party repositories. You should ensure you have necessary rights to share any data that you deposit in a repository.
Data citation
The journal recommends that authors cite any publicly available data on which the conclusions of the paper rely. This includes data the authors are sharing alongside their publication and any secondary data the authors have reused. Data citations should include a persistent identifier (such as a DOI), should be included in the reference list using the minimum information recommended by DataCite (Dataset Creator, Dataset Title, Publisher [repository], Publication Year, Identifier [e.g. DOI, Handle, Accession or ARK]) and follow journal style.
See our further guidance on citing datasets.
Research data and peer review
If the journal that you are submitting to uses double-anonymous peer review and you are providing reviewers with access to your data (for example via a repository link, supplementary information or data on request), it is strongly suggested that the authorship in the data is also anonymised. There are data repositories that can assist with this and/or will create a link to mask the authorship of your data.
Support with research data policy
Authors who need help understanding our data sharing policy, finding a suitable data repository, or organising and sharing research data can consult our Research Data Helpdesk for guidance.
See our FAQ page for more information on Springer Nature’s research data policy.
- Please always use internationally accepted signs and symbols for units ( SI units ).
- Nomenclature: Insofar as possible, authors should use systematic names similar to those used by IUPAC .
- Genus and species names should be in italics.
- Generic names of drugs and pesticides are preferred; if trade names are used, the generic name should be given at first mention.
- Please use the standard mathematical notation for formulae, symbols, etc.: Italic for single letters that denote mathematical constants, variables, and unknown quantities; Roman/upright for numerals, operators, and punctuation, and commonly defined functions or abbreviations, e.g., cos, det, e or exp, lim, log, max, min, sin, tan, d (for derivative); Bold for vectors, tensors, and matrices.
Chemical Abstract Service ( https://www.cas.org/ ) or IUPAC ( https://iupac.org/ )
- Manuscripts submitted to the journal are expected to adhere to internationally accepted nomenclature for receptors ( http://www.guidetopharmacology.org/ ) and enzymes ( https://www.enzyme-database.org/ ).
This journal is committed to upholding the integrity of the scientific record. As a member of the Committee on Publication Ethics ( COPE ) the journal will follow the COPE guidelines on how to deal with potential acts of misconduct.
Authors should refrain from misrepresenting research results which could damage the trust in the journal, the professionalism of scientific authorship, and ultimately the entire scientific endeavour. Maintaining integrity of the research and its presentation is helped by following the rules of good scientific practice, which include*:
- The manuscript should not be submitted to more than one journal for simultaneous consideration.
- The submitted work should be original and should not have been published elsewhere in any form or language (partially or in full), unless the new work concerns an expansion of previous work. (Please provide transparency on the re-use of material to avoid the concerns about text-recycling (‘self-plagiarism’).
- A single study should not be split up into several parts to increase the quantity of submissions and submitted to various journals or to one journal over time (i.e. ‘salami-slicing/publishing’).
- Concurrent or secondary publication is sometimes justifiable, provided certain conditions are met. Examples include: translations or a manuscript that is intended for a different group of readers.
- Results should be presented clearly, honestly, and without fabrication, falsification or inappropriate data manipulation (including image based manipulation). Authors should adhere to discipline-specific rules for acquiring, selecting and processing data.
- No data, text, or theories by others are presented as if they were the author’s own (‘plagiarism’). Proper acknowledgements to other works must be given (this includes material that is closely copied (near verbatim), summarized and/or paraphrased), quotation marks (to indicate words taken from another source) are used for verbatim copying of material, and permissions secured for material that is copyrighted.
Important note: the journal may use software to screen for plagiarism.
- Authors should make sure they have permissions for the use of software, questionnaires/(web) surveys and scales in their studies (if appropriate).
- Research articles and non-research articles (e.g. Opinion, Review, and Commentary articles) must cite appropriate and relevant literature in support of the claims made. Excessive and inappropriate self-citation or coordinated efforts among several authors to collectively self-cite is strongly discouraged.
- Authors should avoid untrue statements about an entity (who can be an individual person or a company) or descriptions of their behavior or actions that could potentially be seen as personal attacks or allegations about that person.
- Research that may be misapplied to pose a threat to public health or national security should be clearly identified in the manuscript (e.g. dual use of research). Examples include creation of harmful consequences of biological agents or toxins, disruption of immunity of vaccines, unusual hazards in the use of chemicals, weaponization of research/technology (amongst others).
- Authors are strongly advised to ensure the author group, the Corresponding Author, and the order of authors are all correct at submission. Adding and/or deleting authors during the revision stages is generally not permitted, but in some cases may be warranted. Reasons for changes in authorship should be explained in detail. Please note that changes to authorship cannot be made after acceptance of a manuscript.
*All of the above are guidelines and authors need to make sure to respect third parties rights such as copyright and/or moral rights.
Upon request authors should be prepared to send relevant documentation or data in order to verify the validity of the results presented. This could be in the form of raw data, samples, records, etc. Sensitive information in the form of confidential or proprietary data is excluded.
If there is suspicion of misbehavior or alleged fraud the Journal and/or Publisher will carry out an investigation following COPE guidelines. If, after investigation, there are valid concerns, the author(s) concerned will be contacted under their given e-mail address and given an opportunity to address the issue. Depending on the situation, this may result in the Journal’s and/or Publisher’s implementation of the following measures, including, but not limited to:
- If the manuscript is still under consideration, it may be rejected and returned to the author.
- an erratum/correction may be placed with the article
- an expression of concern may be placed with the article
- or in severe cases retraction of the article may occur.
The reason will be given in the published erratum/correction, expression of concern or retraction note. Please note that retraction means that the article is maintained on the platform , watermarked “retracted” and the explanation for the retraction is provided in a note linked to the watermarked article.
- The author’s institution may be informed
- A notice of suspected transgression of ethical standards in the peer review system may be included as part of the author’s and article’s bibliographic record.
Fundamental errors
Authors have an obligation to correct mistakes once they discover a significant error or inaccuracy in their published article. The author(s) is/are requested to contact the journal and explain in what sense the error is impacting the article. A decision on how to correct the literature will depend on the nature of the error. This may be a correction or retraction. The retraction note should provide transparency which parts of the article are impacted by the error.
Suggesting / excluding reviewers
Authors are welcome to suggest suitable reviewers and/or request the exclusion of certain individuals when they submit their manuscripts. When suggesting reviewers, authors should make sure they are totally independent and not connected to the work in any way. It is strongly recommended to suggest a mix of reviewers from different countries and different institutions. When suggesting reviewers, the Corresponding Author must provide an institutional email address for each suggested reviewer, or, if this is not possible to include other means of verifying the identity such as a link to a personal homepage, a link to the publication record or a researcher or author ID in the submission letter. Please note that the Journal may not use the suggestions, but suggestions are appreciated and may help facilitate the peer review process.
Authors are requested to disclose interests that are directly or indirectly related to the work submitted for publication. Interests within the last 3 years of beginning the work (conducting the research and preparing the work for submission) should be reported. Interests outside the 3-year time frame must be disclosed if they could reasonably be perceived as influencing the submitted work. Disclosure of interests provides a complete and transparent process and helps readers form their own judgments of potential bias. This is not meant to imply that a financial relationship with an organization that sponsored the research or compensation received for consultancy work is inappropriate.
Editorial Board Members and Editors are required to declare any competing interests and may be excluded from the peer review process if a competing interest exists. In addition, they should exclude themselves from handling manuscripts in cases where there is a competing interest. This may include – but is not limited to – having previously published with one or more of the authors, and sharing the same institution as one or more of the authors. Where an Editor or Editorial Board Member is on the author list we recommend they declare this in the competing interests section on the submitted manuscript. If they are an author or have any other competing interest regarding a specific manuscript, another Editor or member of the Editorial Board will be assigned to assume responsibility for overseeing peer review. These submissions are subject to the exact same review process as any other manuscript. Editorial Board Members are welcome to submit papers to the journal. These submissions are not given any priority over other manuscripts, and Editorial Board Member status has no bearing on editorial consideration.
Interests that should be considered and disclosed but are not limited to the following:
Funding: Research grants from funding agencies (please give the research funder and the grant number) and/or research support (including salaries, equipment, supplies, reimbursement for attending symposia, and other expenses) by organizations that may gain or lose financially through publication of this manuscript.
Employment: Recent (while engaged in the research project), present or anticipated employment by any organization that may gain or lose financially through publication of this manuscript. This includes multiple affiliations (if applicable).
Financial interests: Stocks or shares in companies (including holdings of spouse and/or children) that may gain or lose financially through publication of this manuscript; consultation fees or other forms of remuneration from organizations that may gain or lose financially; patents or patent applications whose value may be affected by publication of this manuscript.
It is difficult to specify a threshold at which a financial interest becomes significant, any such figure is necessarily arbitrary, so one possible practical guideline is the following: "Any undeclared financial interest that could embarrass the author were it to become publicly known after the work was published."
Non-financial interests: In addition, authors are requested to disclose interests that go beyond financial interests that could impart bias on the work submitted for publication such as professional interests, personal relationships or personal beliefs (amongst others). Examples include, but are not limited to: position on editorial board, advisory board or board of directors or other type of management relationships; writing and/or consulting for educational purposes; expert witness; mentoring relations; and so forth.
Primary research articles require a disclosure statement. Review articles present an expert synthesis of evidence and may be treated as an authoritative work on a subject. Review articles therefore require a disclosure statement. Other article types such as editorials, book reviews, comments (amongst others) may, dependent on their content, require a disclosure statement. If you are unclear whether your article type requires a disclosure statement, please contact the Editor-in-Chief.
Please note that, in addition to the above requirements, funding information (given that funding is a potential competing interest (as mentioned above)) needs to be disclosed upon submission of the manuscript in the peer review system. This information will automatically be added to the Record of CrossMark, however it is not added to the manuscript itself. Under ‘summary of requirements’ (see below) funding information should be included in the ‘ Declarations ’ section.
Summary of requirements
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Funding’ and/or ‘Competing interests’. Other declarations include Ethics approval, Consent, Data, Material and/or Code availability and Authors’ contribution statements.
Please see the various examples of wording below and revise/customize the sample statements according to your own needs.
When all authors have the same (or no) conflicts and/or funding it is sufficient to use one blanket statement.
Examples of statements to be used when funding has been received:
- Partial financial support was received from [...]
- The research leading to these results received funding from […] under Grant Agreement No[…].
- This study was funded by […]
- This work was supported by […] (Grant numbers […] and […]
Examples of statements to be used when there is no funding:
- The authors did not receive support from any organization for the submitted work.
- No funding was received to assist with the preparation of this manuscript.
- No funding was received for conducting this study.
- No funds, grants, or other support was received.
Examples of statements to be used when there are interests to declare:
Non-financial interests: Author C is an unpaid member of committee Z.
Non-financial interests: Author A is on the board of directors of Y and receives no compensation as member of the board of directors.
Non-financial interests: none.
Non-financial interests: Author D has served on advisory boards for Company M, Company N and Company O.
Examples of statements to be used when authors have nothing to declare:
- The authors have no relevant financial or non-financial interests to disclose.
- The authors have no competing interests to declare that are relevant to the content of this article.
- All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
- The authors have no financial or proprietary interests in any material discussed in this article.
Authors are responsible for correctness of the statements provided in the manuscript. See also Authorship Principles. The Editor-in-Chief reserves the right to reject submissions that do not meet the guidelines described in this section.
Ethics approval
When reporting a study that involved human participants, their data or biological material, authors should include a statement that confirms that the study was approved (or granted exemption) by the appropriate institutional and/or national research ethics committee (including the name of the ethics committee) and certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. If doubt exists whether the research was conducted in accordance with the 1964 Helsinki Declaration or comparable standards, the authors must explain the reasons for their approach, and demonstrate that an independent ethics committee or institutional review board explicitly approved the doubtful aspects of the study. If a study was granted exemption from requiring ethics approval, this should also be detailed in the manuscript (including the reasons for the exemption).
Retrospective ethics approval
If a study has not been granted ethics committee approval prior to commencing, retrospective ethics approval usually cannot be obtained and it may not be possible to consider the manuscript for peer review. The decision on whether to proceed to peer review in such cases is at the Editor's discretion.
Ethics approval for retrospective studies
Although retrospective studies are conducted on already available data or biological material (for which formal consent may not be needed or is difficult to obtain) ethics approval may be required dependent on the law and the national ethical guidelines of a country. Authors should check with their institution to make sure they are complying with the specific requirements of their country.
Ethics approval for case studies
Case reports require ethics approval. Most institutions will have specific policies on this subject. Authors should check with their institution to make sure they are complying with the specific requirements of their institution and seek ethics approval where needed. Authors should be aware to secure informed consent from the individual (or parent or guardian if the participant is a minor or incapable) See also section on Informed Consent .
If human cells are used, authors must declare in the manuscript: what cell lines were used by describing the source of the cell line, including when and from where it was obtained, whether the cell line has recently been authenticated and by what method. If cells were bought from a life science company the following need to be given in the manuscript: name of company (that provided the cells), cell type, number of cell line, and batch of cells.
It is recommended that authors check the NCBI database for misidentification and contamination of human cell lines. This step will alert authors to possible problems with the cell line and may save considerable time and effort.
Further information is available from the International Cell Line Authentication Committee (ICLAC).
Authors should include a statement that confirms that an institutional or independent ethics committee (including the name of the ethics committee) approved the study and that informed consent was obtained from the donor or next of kin.
Research Resource Identifiers (RRID)
Research Resource Identifiers (RRID) are persistent unique identifiers (effectively similar to a DOI) for research resources. This journal encourages authors to adopt RRIDs when reporting key biological resources (antibodies, cell lines, model organisms and tools) in their manuscripts.
Organism: Filip1 tm1a(KOMP)Wtsi RRID:MMRRC_055641-UCD
Cell Line: RST307 cell line RRID:CVCL_C321
Antibody: Luciferase antibody DSHB Cat# LUC-3, RRID:AB_2722109
Plasmid: mRuby3 plasmid RRID:Addgene_104005
Software: ImageJ Version 1.2.4 RRID:SCR_003070
RRIDs are provided by the Resource Identification Portal . Many commonly used research resources already have designated RRIDs. The portal also provides authors links so that they can quickly register a new resource and obtain an RRID.
Clinical Trial Registration
The World Health Organization (WHO) definition of a clinical trial is "any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes". The WHO defines health interventions as “A health intervention is an act performed for, with or on behalf of a person or population whose purpose is to assess, improve, maintain, promote or modify health, functioning or health conditions” and a health-related outcome is generally defined as a change in the health of a person or population as a result of an intervention.
To ensure the integrity of the reporting of patient-centered trials, authors must register prospective clinical trials (phase II to IV trials) in suitable publicly available repositories. For example www.clinicaltrials.gov or any of the primary registries that participate in the WHO International Clinical Trials Registry Platform .
The trial registration number (TRN) and date of registration should be included as the last line of the manuscript abstract.
For clinical trials that have not been registered prospectively, authors are encouraged to register retrospectively to ensure the complete publication of all results. The trial registration number (TRN), date of registration and the words 'retrospectively registered’ should be included as the last line of the manuscript abstract.
Standards of reporting
Springer Nature advocates complete and transparent reporting of biomedical and biological research and research with biological applications. Authors are recommended to adhere to the minimum reporting guidelines hosted by the EQUATOR Network when preparing their manuscript.
Exact requirements may vary depending on the journal; please refer to the journal’s Instructions for Authors.
Checklists are available for a number of study designs, including:
Randomised trials (CONSORT) and Study protocols (SPIRIT)
Observational studies (STROBE)
Systematic reviews and meta-analyses (PRISMA) and protocols (Prisma-P)
Diagnostic/prognostic studies (STARD) and (TRIPOD)
Case reports (CARE)
Clinical practice guidelines (AGREE) and (RIGHT)
Qualitative research (SRQR) and (COREQ)
Animal pre-clinical studies (ARRIVE)
Quality improvement studies (SQUIRE)
Economic evaluations (CHEERS)
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Ethics approval’.
Examples of statements to be used when ethics approval has been obtained:
• All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of the Medical University of A (No. ...).
• This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University B (Date.../No. ...).
• Approval was obtained from the ethics committee of University C. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
• The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the University of D (Ethics approval number: ...).
Examples of statements to be used for a retrospective study:
• Ethical approval was waived by the local Ethics Committee of University A in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
• This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of XYZ who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRB of XYZ.
• This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of University B approved this study.
Examples of statements to be used when no ethical approval is required/exemption granted:
• This is an observational study. The XYZ Research Ethics Committee has confirmed that no ethical approval is required.
• The data reproduced from Article X utilized human tissue that was procured via our Biobank AB, which provides de-identified samples. This study was reviewed and deemed exempt by our XYZ Institutional Review Board. The BioBank protocols are in accordance with the ethical standards of our institution and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The welfare of animals (vertebrate and higher invertebrate) used for research, education and testing must be respected. Authors should supply detailed information on the ethical treatment of their animals in their submission. For that purpose they may use the ARRIVE checklist which is designed to be used when submitting manuscripts describing animal research.
For studies involving client-owned animals, authors must also document informed consent from the client or owner and adherence to a high standard (best practice) of veterinary care.
Authors are recommended to comply with:
• The International Union for Conservation of Nature (IUCN) Policy Statement on Research Involving Species at Risk of Extinction and consult the IUCN red list index of threatened species .
• Convention on the Trade in Endangered Species of Wild Fauna and Flora
When reporting results authors should indicate:
• … that the studies have been approved by a research ethics committee at the institution or practice at which the studies were conducted. Please provide the name of ethics committee and relevant permit number;
• … whether the legal requirements or guidelines in the country and/or state or province for the care and use of animals have been followed.
Researchers from countries without any legal requirements or guidelines voluntarily should refer to the following sites for guidance:
– The Basel Declaration describes fundamental principles of using animals in biomedical research
– The International Council for Laboratory Animal Science (ICLAS) provides ethical guidelines for researchers as well as editors and reviewers
– The Association for the study of Animal Behaviour describes ethical guidelines for the treatment of animals in research and teaching
– The International Association of Veterinary Editors’ Consensus Author Guidelines on Animal Ethics provide guidelines for authors on animal ethics and welfare
Researchers may wish to consult the most recent (ethical) guidelines available from relevant taxon-oriented professional societies.
If a study was granted exemption or did not require ethics approval, this should also be detailed in the manuscript.
• All procedures involving animals were in compliance with the European Community Council Directive of 24 November 1986, and ethical approval was granted by the Kocaeli University Ethics Committee (No. 29 12 2014, Kocaeli, Turkey).
• All procedures performed in the study were in accordance with the ARVO Statement for Use of Animals in Ophthalmic Vision and Research. The ethical principles established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8523, revised 2011) were followed. The research protocol was approved by the Ethics Committee on Animal Use (Protocol No. 06174/14) of FCAV/Unesp, Jaboticabal.
• This study involved a questionnaire-based survey of farmers as well as blood sampling from their animals. The study protocol was assessed and approved by Haramaya University, research and extension office. Participants provided their verbal informed consent for animal blood sampling as well as for the related survey questions. Collection of blood samples was carried out by veterinarians adhering to the regulations and guidelines on animal husbandry and welfare.
• All brown bear captures and handling were approved by the Ethical Committee on Animal Experiments, Uppsala, Sweden (Application C18/15) and the Swedish Environmental Protection Agency in compliance with Swedish laws and regulations.
• The ethics governing the use and conduct of experiments on animals were strictly observed, and the experimental protocol was approved by the University of Maiduguri Senate committee on Medical Research ethics. Proper permit and consent were obtained from the Maiduguri abattoir management, before the faecal samples of the cattle and camels slaughtered in this abattoir were used for this experiment.
• No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with an unregulated invertebrate species.
• As the trappings of small mammals were conducted as part of regular pest control measures in accordance with the NATO Standardized Agreement 2048 "Deployment Pest and Vector Surveillance and Control ", no approval by an ethics committee was required.
• All experiments have been conducted as per the guidelines of the Institutional Animal Ethics Committee, Department of Zoology, Utkal University, Bhubaneswar, Odisha, India. However, the insect species used in this study is reared for commercial production of raw silk materials, as a part of agro-based industry. Therefore, use of this animal in research does not require ethical clearance. We have obtained permission from the office of Research officer sericulture, Baripada, Orissa, India for the provision of infrastructure and support for rearing of silkworm both in indoor and outdoor conditions related to our study to promote sericulture practices.
All individuals have individual rights that are not to be infringed. Individual participants in studies have, for example, the right to decide what happens to the (identifiable) personal data gathered, to what they have said during a study or an interview, as well as to any photograph that was taken. This is especially true concerning images of vulnerable people (e.g. minors, patients, refugees, etc) or the use of images in sensitive contexts. In many instances authors will need to secure written consent before including images.
Identifying details (names, dates of birth, identity numbers, biometrical characteristics (such as facial features, fingerprint, writing style, voice pattern, DNA or other distinguishing characteristic) and other information) of the participants that were studied should not be published in written descriptions, photographs, and genetic profiles unless the information is essential for scholarly purposes and the participant (or parent/guardian if the participant is a minor or incapable or legal representative) gave written informed consent for publication. Complete anonymity is difficult to achieve in some cases. Detailed descriptions of individual participants, whether of their whole bodies or of body sections, may lead to disclosure of their identity. Under certain circumstances consent is not required as long as information is anonymized and the submission does not include images that may identify the person.
Informed consent for publication should be obtained if there is any doubt. For example, masking the eye region in photographs of participants is inadequate protection of anonymity. If identifying characteristics are altered to protect anonymity, such as in genetic profiles, authors should provide assurance that alterations do not distort meaning.
Exceptions where it is not necessary to obtain consent:
• Images such as x rays, laparoscopic images, ultrasound images, brain scans, pathology slides unless there is a concern about identifying information in which case, authors should ensure that consent is obtained.
• Reuse of images: If images are being reused from prior publications, the Publisher will assume that the prior publication obtained the relevant information regarding consent. Authors should provide the appropriate attribution for republished images.
Consent and already available data and/or biologic material
Regardless of whether material is collected from living or dead patients, they (family or guardian if the deceased has not made a pre-mortem decision) must have given prior written consent. The aspect of confidentiality as well as any wishes from the deceased should be respected.
Data protection, confidentiality and privacy
When biological material is donated for or data is generated as part of a research project authors should ensure, as part of the informed consent procedure, that the participants are made aware what kind of (personal) data will be processed, how it will be used and for what purpose. In case of data acquired via a biobank/biorepository, it is possible they apply a broad consent which allows research participants to consent to a broad range of uses of their data and samples which is regarded by research ethics committees as specific enough to be considered “informed”. However, authors should always check the specific biobank/biorepository policies or any other type of data provider policies (in case of non-bio research) to be sure that this is the case.
Consent to Participate
For all research involving human subjects, freely-given, informed consent to participate in the study must be obtained from participants (or their parent or legal guardian in the case of children under 16) and a statement to this effect should appear in the manuscript. In the case of articles describing human transplantation studies, authors must include a statement declaring that no organs/tissues were obtained from prisoners and must also name the institution(s)/clinic(s)/department(s) via which organs/tissues were obtained. For manuscripts reporting studies involving vulnerable groups where there is the potential for coercion or where consent may not have been fully informed, extra care will be taken by the editor and may be referred to the Springer Nature Research Integrity Group.
Consent to Publish
Individuals may consent to participate in a study, but object to having their data published in a journal article. Authors should make sure to also seek consent from individuals to publish their data prior to submitting their paper to a journal. This is in particular applicable to case studies. A consent to publish form can be found
here. (Download docx, 36 kB)
The above should be summarized in a statement and placed in a ‘Declarations’ section before the reference list under a heading of ‘Consent to participate’ and/or ‘Consent to publish’. Other declarations include Funding, Competing interests, Ethics approval, Consent, Data and/or Code availability and Authors’ contribution statements.
Sample statements for "Consent to participate" :
Informed consent was obtained from all individual participants included in the study.
Informed consent was obtained from legal guardians.
Written informed consent was obtained from the parents.
Verbal informed consent was obtained prior to the interview.
Sample statements for “Consent to publish” :
The authors affirm that human research participants provided informed consent for publication of the images in Figure(s) 1a, 1b and 1c.
The participant has consented to the submission of the case report to the journal.
Patients signed informed consent regarding publishing their data and photographs.
Sample statements if identifying information about participants is available in the article:
Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Images will be removed from publication if authors have not obtained informed consent or the paper may be removed and replaced with a notice explaining the reason for removal.
These guidelines describe authorship principles and good authorship practices to which prospective authors should adhere to.
Authorship clarified
The Journal and Publisher assume all authors agreed with the content and that all gave explicit consent to submit and that they obtained consent from the responsible authorities at the institute/organization where the work has been carried out, before the work is submitted.
The Publisher does not prescribe the kinds of contributions that warrant authorship. It is recommended that authors adhere to the guidelines for authorship that are applicable in their specific research field. In absence of specific guidelines it is recommended to adhere to the following guidelines*:
All authors whose names appear on the submission
1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work;
2) drafted the work or revised it critically for important intellectual content;
3) approved the version to be published; and
4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
* Based on/adapted from:
ICMJE, Defining the Role of Authors and Contributors,
Transparency in authors’ contributions and responsibilities to promote integrity in scientific publication, McNutt at all, PNAS February 27, 2018
Disclosures and declarations
All authors are requested to include information regarding sources of funding, financial or non-financial interests, study-specific approval by the appropriate ethics committee for research involving humans and/or animals, informed consent if the research involved human participants, and a statement on welfare of animals if the research involved animals (as appropriate).
The decision whether such information should be included is not only dependent on the scope of the journal, but also the scope of the article. Work submitted for publication may have implications for public health or general welfare and in those cases it is the responsibility of all authors to include the appropriate disclosures and declarations.
Data transparency
All authors are requested to make sure that all data and materials as well as software application or custom code support their published claims and comply with field standards. Please note that journals may have individual policies on (sharing) research data in concordance with disciplinary norms and expectations.
Role of the Corresponding Author
One author is assigned as Corresponding Author and acts on behalf of all co-authors and ensures that questions related to the accuracy or integrity of any part of the work are appropriately addressed.
The Corresponding Author is responsible for the following requirements:
- ensuring that all listed authors have approved the manuscript before submission, including the names and order of authors;
- managing all communication between the Journal and all co-authors, before and after publication;*
- providing transparency on re-use of material and mention any unpublished material (for example manuscripts in press) included in the manuscript in a cover letter to the Editor;
- making sure disclosures, declarations and transparency on data statements from all authors are included in the manuscript as appropriate (see above).
* The requirement of managing all communication between the journal and all co-authors during submission and proofing may be delegated to a Contact or Submitting Author. In this case please make sure the Corresponding Author is clearly indicated in the manuscript.
Author contributions
In absence of specific instructions and in research fields where it is possible to describe discrete efforts, the Publisher recommends authors to include contribution statements in the work that specifies the contribution of every author in order to promote transparency. These contributions should be listed at the separate title page.
Examples of such statement(s) are shown below:
• Free text:
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [full name], [full name] and [full name]. The first draft of the manuscript was written by [full name] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Example: CRediT taxonomy:
• Conceptualization: [full name], …; Methodology: [full name], …; Formal analysis and investigation: [full name], …; Writing - original draft preparation: [full name, …]; Writing - review and editing: [full name], …; Funding acquisition: [full name], …; Resources: [full name], …; Supervision: [full name],….
For review articles where discrete statements are less applicable a statement should be included who had the idea for the article, who performed the literature search and data analysis, and who drafted and/or critically revised the work.
For articles that are based primarily on the student’s dissertation or thesis , it is recommended that the student is usually listed as principal author:
A Graduate Student’s Guide to Determining Authorship Credit and Authorship Order, APA Science Student Council 2006
Affiliation
The primary affiliation for each author should be the institution where the majority of their work was done. If an author has subsequently moved, the current address may additionally be stated. Addresses will not be updated or changed after publication of the article.
Changes to authorship
Authors are strongly advised to ensure the correct author group, the Corresponding Author, and the order of authors at submission. Changes of authorship by adding or deleting authors, and/or changes in Corresponding Author, and/or changes in the sequence of authors are not accepted after acceptance of a manuscript.
- Please note that author names will be published exactly as they appear on the accepted submission!
Please make sure that the names of all authors are present and correctly spelled, and that addresses and affiliations are current.
Adding and/or deleting authors at revision stage are generally not permitted, but in some cases it may be warranted. Reasons for these changes in authorship should be explained. Approval of the change during revision is at the discretion of the Editor-in-Chief. Please note that journals may have individual policies on adding and/or deleting authors during revision stage.
Author identification
Authors are recommended to use their ORCID ID when submitting an article for consideration or acquire an ORCID ID via the submission process.
Deceased or incapacitated authors
For cases in which a co-author dies or is incapacitated during the writing, submission, or peer-review process, and the co-authors feel it is appropriate to include the author, co-authors should obtain approval from a (legal) representative which could be a direct relative.
Authorship issues or disputes
In the case of an authorship dispute during peer review or after acceptance and publication, the Journal will not be in a position to investigate or adjudicate. Authors will be asked to resolve the dispute themselves. If they are unable the Journal reserves the right to withdraw a manuscript from the editorial process or in case of a published paper raise the issue with the authors’ institution(s) and abide by its guidelines.
Confidentiality
Authors should treat all communication with the Journal as confidential which includes correspondence with direct representatives from the Journal such as Editors-in-Chief and/or Handling Editors and reviewers’ reports unless explicit consent has been received to share information.
Manuscripts that report experiments involving the use of human embryos and gametes, human embryonic stem cells and related materials, and clinical applications of stem cells must include confirmation that all experiments were performed in accordance with relevant guidelines and regulations (See also Research involving human participants, their data or biological material .
The manuscript should include an ethics statement identifying the institutional and/or national research ethics committee (including the name of the ethics committee) approving the experiments and describing any relevant details. Authors should confirm that informed consent (See also Informed Consent ) was obtained from all recipients and/or donors of cells or tissues, where necessary, and describe the conditions of donation of materials for research, such as human embryos or gametes. Copies of approval and redacted consent documents may be requested by the Journal.
We encourage authors to follow the principles laid out in the ISSCR Guidelines for Stem Cell Research and Clinical Translation .
In deciding whether to publish papers describing modifications of the human germline, the Journal is guided by safety considerations, compliance with applicable regulations, as well as the status of the societal debate on the implications of such modifications for future generations. In case of concerns regarding a particular type of study the Journal may seek the advice from the Springer Nature Research Integrity Group.
The decision to publish a paper is the responsibility of the Editor-in-Chief of the Journal.
This journal values stewardship, transparency, and adhering to governance with regards to collecting and utilizing specimens and conducting experiments and/or field studies. Therefore the journal sets out the following guidelines:
Field studies involving genetically engineered plants must be conducted in accordance with national or local legislation and, if applicable, the manuscript needs to include a statement specifying the appropriate permissions and/or licences.
Authors utilizing genetic plant resources received via local suppliers/collectors, such as species collected from protected areas or endangered species with medical importance, must conduct their experiments following the Nagoya Protocol (as part of the Convention on Biological Diversity).
Authors whose research is focusing on quarantine organisms (i.e. harmful or pest organisms, including plant pathogens) should adhere to national legislation and notify the relevant National Plant Protection Organization of new findings before publication. More information can be found via the International Plant Protection Convention .
In principle, it is recommended that authors comply with:
- The International Union for Conservation of Nature (IUCN) Policy Statement on Research Involving Species at Risk of Extinction and consult the IUCN red list index of threatened species
- Convention on the Trade in Endangered Species of Wild Fauna and Flora
Voucher specimens ensure that the identity of organisms studied in the field or in laboratory experiments can be verified, and ensure that new species concepts can be applied to past research. Voucher specimens documenting all investigated accessions (for population samples at least one specimen per population) are to be deposited in a public herbarium, for example: Index Herbariorum , or other public collection providing access to deposited material. Information on the voucher specimen and who identified it must be included in the manuscript such as Genus name, species name, author, and year of publication.
Names of plants, algae and fungi
Manuscripts containing new taxon names or other nomenclatural acts must follow the guidelines set by the International Code of Nomenclature for algae, fungi, and plants .
Authors describing new fungal taxa should register the names with a recognized repository, such as Mycobank , and request a unique digital identifier which should be included in the published article.
Single-blind peer review
This journal follows a single-blind reviewing procedure.
This journal also publishes special/guest-edited issues. The peer review process for these articles is the same as the peer review process of the journal in general.
Additionally, if a guest editor authors an article in their issue/collection, they will not handle the peer review process.
Additional Information
All submissions to Springer journals are first reviewed for completeness and only then sent to be assessed by an Editor who will decide whether they are suitable for peer review. Where an Editor is on the author list or has any other competing interest regarding a specific manuscript, another member of the Editorial Board will be assigned to oversee peer review. Editors will consider the peer-reviewed reports when making a decision, but are not bound by the opinions or recommendations therein. A concern raised by a single peer reviewer or the Editor themself may result in the manuscript being rejected. Authors receive peer review reports with the editorial decision on their manuscript.
This journal operates a single-blind peer-review system, where the reviewers are aware of the names and affiliations of the authors, but the reviewer reports provided to authors are anonymous.The benefit of single-blind peer review is that it is the traditional model of peer review that many reviewers are comfortable with, and it facilitates a dispassionate critique of a manuscript.
Submitted manuscripts will generally be reviewed by two or more experts who will be asked to evaluate whether the manuscript is scientifically sound and coherent, whether it duplicates already published work, and whether or not the manuscript is sufficiently clear for publication. The Editors will reach a decision based on these reports and, where necessary, they will consult with members of the Editorial Board.
When selecting reviewers, the editorial board seeks to avoid conflicts of interest. The journal accepts manuscript submissions from its own editorial board members in cases where the identities of the associate editor and referees handling the manuscript can remain fully confidential. To be accepted, manuscripts submitted by editorial board members must meet the same quality standards as all other accepted submissions.
How can you help improve your manuscript for publication?
Presenting your work in a well-structured manuscript and in well-written English gives it its best chance for editors and reviewers to understand it and evaluate it fairly. Many researchers find that getting some independent support helps them present their results in the best possible light. The experts at Springer Nature Author Services can help you with manuscript preparation—including English language editing, developmental comments, manuscript formatting, figure preparation, translation , and more.
Get started and save 15%
You can also use our free Grammar Check tool for an evaluation of your work.
Please note that using these tools, or any other service, is not a requirement for publication, nor does it imply or guarantee that editors will accept the article, or even select it for peer review.
Chinese (中文)
您怎么做才有助于改进您的稿件以便顺利发表?
如果在结构精巧的稿件中用精心组织的英语展示您的作品,就能最大限度地让编辑和审稿人理解并公正评估您的作品。许多研究人员发现,获得一些独立支持有助于他们以尽可能美好的方式展示他们的成果。Springer Nature Author Services 的专家可帮助您准备稿件,具体包括 润色英语表述、添加有见地的注释、为稿件排版、设计图表、翻译 等。
开始使用即可节省 15% 的费用
您还可以使用我们的 免费语法检查工具 来评估您的作品。
请注意,使用这些工具或任何其他服务不是发表前必须满足的要求,也不暗示或保证相关文章定会被编辑接受(甚至未必会被选送同行评审)。
Japanese (日本語)
発表に備えて、論文を改善するにはどうすればよいでしょうか?
内容が適切に組み立てられ、質の高い英語で書かれた論文を投稿すれば、編集者や査読者が論文を理解し、公正に評価するための最善の機会となります。多くの研究者は、個別のサポートを受けることで、研究結果を可能な限り最高の形で発表できると思っています。Springer Nature Author Servicesのエキスパートが、 英文の編集、建設的な提言、論文の書式、図の調整、翻訳 など、論文の作成をサポートいたします。
今なら15%割引でご利用いただけます
原稿の評価に、無料 の文法チェック ツールもご利用いただけます。
これらのツールや他のサービスをご利用いただくことは、論文を掲載するための要件ではありません。また、編集者が論文を受理したり、査読に選定したりすることを示唆または保証するものではないことにご注意ください。
Korean (한국어)
게재를 위해 원고를 개선하려면 어떻게 해야 할까요?
여러분의 작품을 체계적인 원고로 발표하는 것은 편집자와 심사자가 여러분의 연구를 이해하고 공정하게 평가할 수 있는 최선의 기회를 제공합니다. 많은 연구자들은 어느 정도 독립적인 지원을 받는 것이 가능한 한 최선의 방법으로 자신의 결과를 발표하는 데 도움이 된다고 합니다. Springer Nature Author Services 전문가들은 영어 편집, 발전적인 논평, 원고 서식 지정, 그림 준비, 번역 등과 같은 원고 준비를 도와드릴 수 있습니다.
지금 시작하면 15% 할인됩니다.
또한 당사의 무료 문법 검사 도구를 사용하여 여러분의 연구를 평가할 수 있습니다.
이러한 도구 또는 기타 서비스를 사용하는 것은 게재를 위한 필수 요구사항이 아니며, 편집자가 해당 논문을 수락하거나 피어 리뷰에 해당 논문을 선택한다는 것을 암시하거나 보장하지는 않습니다.
To find out more about publishing your work Open Access in Medicinal Chemistry Research , including information on fees, funding and licences, visit our Open access publishing page .
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: October 2004
A guide to drug discovery
The role of the medicinal chemist in drug discovery — then and now
- Joseph G. Lombardino 1 &
- John A. Lowe III 2
Nature Reviews Drug Discovery volume 3 , pages 853–862 ( 2004 ) Cite this article
74k Accesses
237 Citations
21 Altmetric
Metrics details
Medicinal chemists paly a crucial role in the drug discovery process through the selection and synthesis of compounds that establish structure–activity relationships and achieve efficacy and safety in preclinical animal testing
Many aspects of the medicinal chemist's role have changed since the early era of drug discovery when animal testing and small, informal project teams dominated the process.
Combinatorial chemistry, high-throughput screening and molecularly defined targets that allow structure-based drug design have changed the chemist's role in the modern era.
In vitro screens for pharmacokinetic properties, the focus on synthesizing drug-like compounds, and in vitro toxicity screens are important new developments that aid the medicinal chemist's job today.
Suggestions for improving the drug discovery process include more in vivo testing earlier in the drug discovery process, allowing medicinal chemists to champion their drug candidate during its development; and passing on the tacit knowledge of experienced medicinal chemists to their younger colleagues.
The role of the medicinal chemist in drug discovery has undergone major changes in the past 25 years, mainly because of the introduction of technologies such as combinatorial chemistry and structure-based drug design. As medicinal chemists with more than 50 years of combined experience spanning the past four decades, we discuss this changing role using examples from our own and others' experience. This historical perspective could provide insights in to how to improve the current model for drug discovery by helping the medicinal chemist regain the creative role that contributed to past successes.
Similar content being viewed by others
The Pan-Canadian Chemical Library: A Mechanism to Open Academic Chemistry to High-Throughput Virtual Screening
Molecular chameleons in drug discovery

Natural products in drug discovery: advances and opportunities
Medicinal chemists prepare and/or select appropriate compounds for biological evaluation that, if found to be active, could serve as LEAD COMPOUNDS . They then evaluate the STRUCTURE–ACTIVITY RELATIONSHIPS (SARs) of analogous compounds with regard to their in vitro and in vivo efficacy and safety. Today, medicinal chemists who are engaged in drug discovery are part of interdisciplinary teams, and must therefore understand not only the field of organic chemistry, but also a range of other disciplines to anticipate problems and interpret developments to help move the project forward.
As highlighted in this article, the role of the medicinal chemist has changed significantly in the past 25 years. In the early era ('then') of drug discovery (1950 to about 1980), medicinal chemists relied primarily on data from in vivo testing. In the more recent ('now') period (about 1980 to the present), the development of new technologies, such as high-throughput in vitro screening, large compound libraries, COMBINATORIAL TECHNOLOGY , defined molecular targets and structure-based drug design, has changed that earlier and relatively simple landscape. Although these new technologies present many opportunities to the medicinal chemist, the multitude of new safety requirements that have arisen has also brought unanticipated hurdles for the task of translating in vitro activity to in vivo activity. Simultaneously, the knowledge base that supports drug research has expanded considerably, increasing the challenge for chemists to understand their fields of expertise. The demonstration of adequate clinical safety and efficacy in humans has also become more complex, and ever-increasing amounts of data are now required by regulatory agencies. In fact, despite the use of many new technologies, and the growing resources and funding for drug research, the number of launches of new medicines in the form of NEW MOLECULAR ENTITIES (NMEs) has been generally decreasing for more than a decade. Clearly, the difficulty and complexity of drug research has increased in the past two decades. It is our aim with this article to discuss how these changes have influenced the role of medicinal chemists and to suggest ways to help them to contribute more effectively to the drug discovery process.
The process of drug discovery
Inventing and developing a new medicine is a long, complex, costly and highly risky process that has few peers in the commercial world. Research and development (R&D) for most of the medicines available today has required 12–24 years for a single new medicine, from starting a project to the launch of a drug product ( Fig. 1 ). In addition, many expensive, long-term research projects completely fail to produce a marketable medicine. The cost for this overall process has escalated sharply to up to an estimated US $1.4 billion for a single new drug 1 . All of the funds to support this research usually come from the income of the private pharmaceutical company that sponsors the work. In the research ('R'; discovery) phase, only a fraction of the scientific hypotheses that form the basis for a project actually yield a drug candidate for development. In the drug development ('D') phase, experience has shown that only approximately 1 out of 15–25 drug candidates survives the detailed safety and efficacy testing (in animals and humans) required for it to become a marketed product. And for the few drug candidates that successfully become marketed products, some will not recover their costs of development in the competitive marketplace, and only approximately one in three will become a major commercial product. Clearly, this is a high-stakes, long-term and risky activity, but the potential benefits to the millions of patients with serious diseases provide a constant motivating force. At virtually every phase — from project initiation to discovery, development and planning for marketing for a new drug — the modern medicinal chemist can have a role.
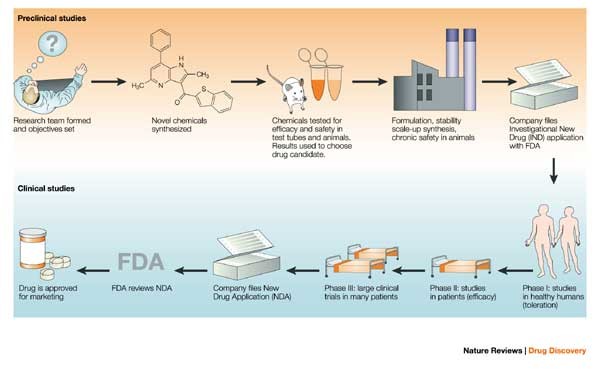
The drug discovery process begins with the identification of a medical need, including a judgement on the adequacy of existing therapies (if there are any). From this analysis, together with an appraisal of the current knowledge about the target disease, will come hypotheses on how to possibly improve therapy — that is, what efficacy, safety or mechanistically novel improvements will advance the method of drug treatment for patients with the target disease? On the basis of these hypotheses, specific objectives will be set for the project. Then, testing selected chemicals in appropriate biological tests can begin. Key subsequent steps in the process include detecting relevant biological activity (a 'hit') for a structurally novel compound in vitro , then finding a related compound with in vivo activity in an appropriate animal model, followed by maximizing this activity through the preparation of analogous structures, and finally selecting one compound as the drug development candidate. This drug candidate then undergoes toxicological testing in animals, as required by law. If the compound passes all these tests, all the accumulated research data are assembled and submitted as an Investigational New Drug Application (IND) to the Food and Drug Administration (FDA) in the United States (or comparable agency in other countries) before clinical trials are initiated. In the clinic, there is sequential evaluation in normal human volunteers of toleration (Phase I), efficacy and dose range in patients (Phase II), followed by widespread trials in thousands of appropriate patients to develop a broad database of efficacy and safety. For the few (4–7%) drug candidates that survive this series of development trials, a New Drug Application (NDA) that contains all the accumulated research data is filed for thorough review by the experts at the FDA. Only with their approval can the new drug be offered to doctors and their patients to treat the disease for which it was designed.
The role of the medicinal chemist
The modern medicinal chemist, although part of a team, has a particularly crucial role in the early phases of drug discovery. The chemist, trained to prepare new chemicals and with an acquired knowledge of the target disease and of competitive drug therapies, has an important part in framing the hypothesis for the new drug project, which then sets the objectives for the project. The chemist also helps to decide which existing chemicals to screen for a lead compound and which screening hits need to be re-synthesized for biological evaluation. Purification and proper characterization of the new chemicals is also the responsibility of the chemist. When an in vitro ' HIT ' is identified, the chemist decides on what analogous compounds should be obtained or synthesized to explore the SARs for the structural family of compounds in an effort to maximize the desired activity. Developing in vivo activity for the hit compound in an appropriate animal model is also mainly the responsibility of the chemist. This can often be one of the most difficult steps to accomplish because several factors, such as absorbability, distribution in vivo , rate of metabolism and rate of excretion (ADME), all present hurdles for the chemist to solve in the design and preparation of new, analogous chemicals for testing. The goal at this stage is to maximize efficacy while minimizing side effects in an animal model.
For the medicinal chemist to address all the challenges outlined above, several skills are required. These include a thorough knowledge of modern organic chemistry and medicinal chemistry, an understanding of the biology that relates to the target disease, an understanding of the pharmacological tests used in the project and sufficient knowledge of the factors that influence ADME characteristics of chemicals in vivo . Furthermore, they should also have an understanding of clinical medicine that pertains to the target disease; knowledge of the regulatory requirements for related drugs; a current knowledge of competitive therapies, both in the market and under development by competitors; a thorough knowledge of the literature that is relevant to the target disease; familiarity with the many newer technologies available to facilitate drug discovery; and an entrepreneurial attitude in behaving as an innovator and inventor. Finally — and of crucial importance to the timely success of the project — the chemist must show superior interpersonal skills throughout the life of the project to interact effectively with colleagues from other disciplines to achieve project goals.
The medicinal chemist — then and now
Then (1950s–1980s). About 25–45 years ago, a medicinal chemist's tasks differed in some ways from those of a chemist today; an example of a successful project from this era (the development of the anti-inflammatory agent piroxicam (Feldene; Pfizer)) is highlighted in Box 1 . At that time, the medicinal chemist and a pharmacologist counterpart were the main drivers of the research programme: compounds were designed and individually synthesized by the chemist in gram quantities to accommodate the need for testing in whole animals by the pharmacologist. Given the limited synthetic methodology available, these syntheses were often time-consuming and, even with one or two technical assistants working in the laboratory, the output from one chemistry laboratory was limited to an average of one to three compounds per week. Commercially available starting materials were often limited. The chemist had only a few tools (for example, infrared and ultraviolet spectroscopy, and column chromatography) to assist with compound characterization and purification. Outsourcing was rare; all tasks, including bulk syntheses, toxicological testing and analogue synthesis, were done in-house. The creativity and intuition of the medicinal chemist was pivotal to the success of the programme, although given the limited number of compounds produced, serendipity had a large role as well.
Projects generally used in vivo models for primary screening, as little was known about the detailed biological mechanisms involved in most diseases. In vitro testing against a key enzyme or specific receptor involved in the disease process was usually not possible; as discussed in Box 2 (which describes the discovery of the antipsychotic ziprasidone (Geodon; Pfizer)), in vitro receptor-based pharmacology only became common in the 1980s and 1990s. In addition, compound collections for exploratory biological screening were limited. The data generated from the test models were compiled, analysed and displayed by hand in the form of charts and graphs. Similarly, searching the literature for relevant information involved the handling of bound volumes taken individually from the library shelves.
Small companies tend to rely on informal communication and timelines, and this was often the case in the smaller pharmaceutical industry 'then'. For the medicinal chemist, the benefit of this informality was ready access to colleagues in other disciplines to evaluate a compound that the chemist was interested in. The disadvantage came once a chemist's compound was selected for further development. The chemist, who would probably have moved on to another project, usually heard little or nothing about the drug candidate until the (often) bad news came back that the candidate had failed some key test. Keeping abreast of the progress of the drug candidate required the same proactive, informal action that the chemist had used previously to periodically contact the appropriate scientists in other disciplines to get some news about the drug candidate. To address these issues, most organizations in the 1980s established interdisciplinary matrix teams for each drug candidate to facilitate information exchange and joint planning between departments, such as chemistry, biology, pharmaceutics, toxicology, PHARMACOKINETICS , clinical medicine and regulatory affairs, all of which have important roles in drug development.
Overall, the process of drug discovery 'then' was slower and operated from a relatively smaller knowledge base. Several factors combined to slow the process: there was less known about diseases, there were fewer available compounds to screen, there were no computerized technologies for handling information and data, there was a need to manually search the literature, there was a need to individually prepare gram quantities of each new compound for testing, and chemists rarely received information from other disciplines about their development candidates. On the other hand, once a lead was identified in the primary in vivo test model, many of the pharmacokinetic (ADME) problems were mainly in hand or could be rapidly addressed, thereby expediting the selection of a drug candidate to study in the clinic.
Now (1980s–present). Despite some differences from the earlier era of drug discovery described above, medicinal chemists today face many of the same tasks and challenges that they did 40 years ago. So, the chemist still selects the appropriate structural series of compounds to follow and pursues the SARs to identify suitable drug candidates for advancement to safety and clinical testing. But today's chemist has a much wider range of tools to help overcome the numerous hurdles in the drug discovery process. These new tools include advances in synthetic, analytical and purification technology, such as transition-metal-catalysed carbon–carbon bond-forming reactions, high-field NMR and preparative high-performance liquid chromatography (HPLC), as well as computer-assisted literature and data retrieval and analysis. The recent trend towards outsourcing many routine, tedious aspects of the drug discovery process has freed today's chemist to spend more time on new compound design. In addition, two powerful technologies have put numbers on the chemist's side: combinatorial chemistry (combichem) and high-throughput screening (HTS). Combichem allows chemists to generate rational, focused libraries of compounds that define SARs in a fraction of the time that was required 'then'. Depending on where they work, chemists can design, synthesize and purify libraries themselves, or hand over the final synthesis steps to a group of chemists designated for this purpose. This group might also make lead-compound libraries that target specific receptor or enzyme families to provide better quality leads that are suitable for library follow up. The development of HTS of large sample collections, including the designed libraries, has produced marked decreases in the personnel, time and money required to identify compounds that hit a specific biological target, although many companies are struggling to triage the large number of screening hits to viable lead compounds that can support a successful drug discovery project. In this struggle, costs can escalate significantly as the generation of large amounts of data is not the same as generating viable, quality leads. Finally, new graphics software, such as Excel and Spotfire 2 , can facilitate the retrieval and analysis of the mountain of data generated from screening compound libraries in a large panel of in vitro assays.
The molecular genetics revolution 3 has driven the development of another key ingredient in today's drug discovery model: the use of molecularly defined biological targets, such as enzymes, receptors and transporters. The desire for defined molecular targets for drug discovery, in contrast to the clinically based animal-model approach used in the early era of drug discovery discussed above, derives from several factors. One is the advantage of a known mechanism of action over a 'black-box' (that is, unknown) mechanism obtained from animal-model testing that could produce unanticipated toxicity during drug development. Another is the use of structure-based drug design, which allows the chemist to design new compounds by directly visualizing the interaction of a lead compound with the target protein through X-ray crystallographic analysis, but which is only possible with a molecularly defined target protein 4 . A recent example from the new era of drug discovery described in Box 3 (the kinase inhibitor imatinib mesylate (Gleevec; Novartis)) illustrates these advantages, which are now so well established that retreat to the black-box models of yesteryear is no longer feasible.
Recent changes — medicinal chemistry today
New techniques for addressing pharmacokinetic issues. The emphasis on in vitro screening of compounds against molecularly defined targets, although rapid and specific, has additional consequences for today's medicinal chemist. As the primary screen used to guide SAR studies, in vitro data do not help chemists to overcome the pharmacokinetic liabilities of their compounds. On the other hand, relying on in vivo animal models for the evaluation of pharmacokinetic performance suffers from a potentially serious drawback: differences between absorption and metabolism of drugs in humans and rats (a common test species) can lead to the development of drugs that work only in rats and not in humans. To help overcome this limitation, in vitro screens have been developed that are predictive of human pharmacokinetic performance, for example, by measuring a compound's degradation by preparations of human microsomes or hepatocytes or by recombinant human CYTOCHROME P450 ENZYMES . In addition to assessing metabolic stability, P450 assays can determine whether a compound is likely to interfere with the metabolism of other drugs that a patient is taking by virtue of inhibiting the P450 enzyme required for their elimination. Permeability and transporter assays have also been developed to characterize drug uptake into or efflux from the target organ(s) (for a review of the P-glycoprotein (Pgp) transporter in drug development, see Ref. 5 ). So, today's chemist has a complex array of in vitro SAR patterns to discern and interpret to plan the preparation of compounds for follow up (for a review of the screening data typically used in the drug discovery process, see Ref. 6 ). Selected compounds must also be profiled in vivo to assess how well the in vitro data predict in vivo performance. Further in vivo testing is then required to show that the compound attains levels at the target organ commensurate with achieving the desired biological effect that is proposed to result from the in vitro activity.
Final testing might involve a disease-relevant animal model, although these data must be interpreted cautiously owing to several limitations. For example, many diseases, such as stroke , atherosclerosis and Alzheimer's disease , do not have clinically effective drugs that can validate a disease-progression-relevant animal model. Also, older models are based on drugs that work by certain mechanisms, and might not fairly assess drugs that are developed against a new mechanism. As such, the disease-relevant animal model is only one of many assays used to evaluate new compounds and, coming later in the testing sequence, has less impact on decisions made by today's chemists.
Synthesis of 'drug-like' compounds. Another strategy to overcome pharmacokinetic liabilities is the prediction and synthesis of compounds with ' DRUG-LIKE ' properties. Highly lipophilic, high-molecular-mass compounds tend to have more potent in vitro binding activity, by virtue of excluding water from the enzyme or receptor surface and thereby picking up additional hydrophobic interactions. But these compounds are usually not drug-like because of their low water solubility, and they generally fail in further development because of poor pharmacokinetics and oral BIOAVAILABILITY . Lipinski et al . 7 formulated the 'rule-of-five' to predict drug-likeness, which consists of four important properties, each related to the number 5 (molecular mass <500 Da; calculated LOGP <5; hydrogen-bond donors <5; and hydrogen-bond acceptors <10). The rule is based on data in the literature for a large number of compounds, including all known drugs, that correlate physical properties with oral bioavailability. Support for the rule as a predictor of drug-likeness comes from observing weaknesses in the development pipelines of major pharmaceutical companies owing to failure to adhere to the rule-of-five 8 . Computational calculations routinely predict rule-of-five properties for prospective compounds in a chemist's SAR plans to guide compound selection, although this guidance comes at the cost of adding complexity to an already complex set of in vitro data.
Use of in vitro toxicity screens to reduce attrition. Completing the in vitro screens that the chemist uses to select the next compound to synthesize are the toxicity screens that weed out compounds predicted to fail for safety reasons. The Ames test, and related in vitro tests for mutagenicity and carcinogenicity, has a long history, but recent additions to this list include the hERG channel, a cardiac potassium ion channel involved in cardiac repolarization following ventricle contraction during the heartbeat 9 . Drugs that bind to and inhibit the hERG channel can cause prolongation of the QT interval of the electrocardiogram, leading to loss of a synchronous heartbeat and eventually ventricular fibrillation, and even death. The danger posed by a drug that inhibits the hERG channel was illustrated by the deaths of patients taking the allergic rhinitis drug astemizole (Hismanal; Janssen), which led to its abrupt withdrawal from the market 10 . In the aftermath of this and other incidents of fatal complications from hERG-blocking drugs, the FDA is formulating guidelines to address the issue. Most pharmaceutical companies now have hERG screening in place to afford chemists an indication of the therapeutic index of their compounds for this end point 11 .
Box 4 summarizes the various criteria that today's chemist must follow to develop a successful drug candidate. A recent literature example that illustrates many of the new techniques and testing hurdles for today's medicinal chemist — a series of farnesyl transferase inhibitors — is given in Box 5 .
Final thoughts on the drug discovery process
The role of a champion in drug discovery. As a scientist involved at the very earliest stages of drug discovery, including the setting of project objectives, the medicinal chemist with leadership qualities has the opportunity to act as a champion for the drug candidate throughout the long R&D process. Championing a drug candidate was often a key factor in a successful drug project 'then' and was facilitated by the smaller project teams typical of this earlier era. For example, key publications concerning a new drug often had just two authors, the chemist and the biologist, who were essentially the drug champions. There is a multitude of commercially successful drugs today that survived a dark period during development only because a champion worked to keep the drug alive by finding answers to problems (see examples provided in Ref. 12 ).
To act as a champion for a drug candidate, a chemist with current knowledge of all aspects of the drug programme must take an enduring, pervasive interest in all aspects of the development process, especially in helping to solve those seemingly intractable challenges that inevitably arise during the long path to regulatory approval. Without a champion, a drug candidate can lose momentum and stall irreversibly during the years leading to regulatory approval. This is truer today than ever, because the process has become so much more complicated. And yet the contribution of a medicinal chemist can seem diluted by the presence of scientists from the many other disciplines that make up a typical drug discovery programme today, disciplines which have risen significantly in importance in recent years. In addition to the increased number of contributing scientific sub-specialities today, the high cost and increased complexity of drug R&D today 1 can dwarf any one scientist's contribution.
Suggestions for improving the drug discovery process. Recent data indicate that productivity has not kept pace with increasing resource allocation to the drug discovery process. We would like to suggest three ways to improve the current model for new drug discovery that would help the medicinal chemist to be more productive. The first stems from the current heavy reliance on in vitro screening for driving SARs early in a programme, at the risk of finding poor pharmacokinetics and oral bioavailability later on. Coordinating animal testing with in vitro testing early in the drug discovery process to pre-screen lead series in vivo , and then correlating in vitro pharmacokinetics screens with in vivo data as soon as possible, might provide a firmer footing for the chemist to overcome any deficiencies in pharmacokinetics. Such testing might also help to identify lead compounds on the basis of their promising in vivo activity or pharmacokinetic properties that would have been rejected on the basis of in vitro testing alone.
The second suggestion is based on the need to have a committed drug champion to bring background information and a historical perspective (sometimes termed 'institutional memory'), and to suggest solutions to the myriad issues that arise throughout a drug's development. By appointing a small, permanent committee, which includes the medicinal chemist from the discovery team, to be involved with the entire drug development programme through to drug registration (and to work alongside the interdisciplinary matrix development teams), there would always be someone available to provide informed judgments on the basis of their medicinal chemistry background and experience on the project to help keep the drug on track during the many years required for its successful development.
Finally, as many of the most experienced chemists in the pharmaceutical industry reach retirement age, there remains the challenge of how to pass on their learning to the next generation. They possess tacit knowledge (that is, residing in the mind of the experienced scientist but not yet communicated to others) of the drug discovery experience that needs to be recognized, captured and then passed on to the young scientists (as outlined in Ref. 13 ). Companies that accomplish this, by, for example, holding in-house workshops on drug design and lecture series on medicinal chemistry, will help to teach the next generation of scientists the art of successful drug discovery.
The changing landscape of the pharmaceutical industry. Some basic questions about the new technologies and procedures now used for drug research, compared with the dwindling supply of new drugs approved in recent years, have been raised in recent news articles 14 , 15 , 16 , 17 . For example, has the introduction of major changes in the drug discovery process caused the obvious drop in new drug output? Is this drop temporary, to last only until the new technologies begin to yield some products? Have the changes produced a decrease in output by stifling the creativity of the scientists (including the medicinal chemists) involved in drug discovery? Has the role of serendipity, so important to drug discovery in the past, been supplanted by robots? What has happened to the role of the medicinal chemist's intuition and creativity in producing quality drugs? How many of today's most successful drugs could have been made through the limited chemical pathways offered by combichem techniques? Making millions of new chemicals robotically does not, apparently, lead to more new drugs.
An important perspective on this discussion comes from a recent account 17 of the key differences in the pharmaceutical industry experienced by a father–son pair of medicinal chemists, Leo Sternbach ('then', about 40–50 years ago, when he invented chlordiazepoxide (Librium; Hoffman-La Roche) and diazepam (Valium; Hoffman-La Roche)) and his son, Daniel ('now', currently a medicinal chemist at GlaxoSmithKline). By their account, the role of the medicinal chemist has changed considerably from that of a highly autonomous, independent inventor 'then' to a significant player in a large team that is increasingly influenced by the business units 'now'.
In our opinion, whatever the merits of the business decisions that led to this change, the role of serendipity, chemical intuition and creativity in thoughtfully selecting a chemical target to synthesize in order to discover the best-quality drugs has not diminished. There must always be an opportunity in research for the useful chance observation by a prepared mind. There are many examples of 'back burner' (that is, unauthorized) projects that have yielded important new drugs. Although the new technologies that have accelerated the process of drug discovery provide some undoubted benefits, the human factor remains an integral part of success in this endeavour. It is our hope that the accounts of successful drug discoveries presented here will serve as a reminder of the chemists whose decisions actually led to these success stories.
Today, the rapidly expanding knowledge base concerning diseases, their causes, symptoms and their effects on the human body holds great promise for the discovery of important new medicines. Sequencing the human genome also offers the opportunity for finding many more novel and selective therapies. Such discoveries will probably come from teams of scientists, including medicinal chemists, whose careers are devoted to this one task. The enormous cost of this task will be borne mainly by those pharmaceutical companies that can successfully generate the required research funds from the sale of their existing drugs.
Medicinal chemists today live in exciting times. They are key participants in the effort to produce more selective, more effective and safer medicines to treat the diseases of mankind. Their work can have a beneficial effect on millions of suffering patients — surely an important motivating factor for any scientist.
Box 1 | Discovery of piroxicam (1962–1980)
The project that produced the novel anti-arthritic and anti-inflammatory agent piroxicam (Feldene; Pfizer) began in 1962 and led to the product launching into key European markets in 1980. A detailed history of this 18-year process, including the failures and setbacks along the way, has been described elsewhere 12 , 18 , so only a brief outline will be given here.
The original research team assigned to produce a new anti-inflammatory agent at Pfizer consisted of just two people — a medicinal chemist and a pharmacologist. Both were new to the area of inflammation research and had to educate themselves on all aspects of this therapeutic area. Several therapies for treating the symptoms of arthritis were already available or in development at other companies. These therapies included aspirin, indomethacin, diclofenac, ibuprofen and others. The medicinal chemist noted that all of these agents were from one chemical class — the carboxylic acids. Members of this chemical class were known to be rapidly metabolized and excreted, therefore necessitating multiple daily dosing (three to six times a day) of these drugs to maintain control of the pain and swelling of arthritis. These multiple daily doses were a feature that patients found to be undesirable and led to poor compliance. Furthermore, high daily doses (up to 16 g of aspirin) were required for some of these relatively non-potent agents, therefore placing a heavy load on the gastrointestinal tract, liver and kidneys, and consequently increasing the potential for toxicity.
In the early period of the project that eventually produced piroxicam, a set of project objectives were gradually developed that guided the project in the succeeding years. These objectives were to:
seek a structurally novel compound with acidic properties, but not a carboxylic acid.
seek a highly potent anti-inflammatory agent in animal models that was predictive of clinical activity and to use as controls the drugs known to be efficacious in humans.
identify an active agent that resists metabolism that would produce a long plasma half-life in animals and in humans, and consequently lead to reduced frequency of dosing in humans.
seek a very safe agent that arthritic patients could use over long periods of time to treat their chronic disease.
These stringent objectives placed formidable hurdles in the pathway to success and prolonged the time required to successfully achieve the goal.
The synthesis of gram quantities of compounds designed by the chemist then began, all of which were thought to have the potential to fulfill the project objectives. The acidity (p K a ) of each structure was measured and the serum half-life in dogs was determined for selected analogues to guide the synthesis programme. Using in vivo animal models of inflammation (this was before prostaglandins were known to be involved in inflammation), several families of compounds were found and partially developed ( a–d ), but each failed during a 5-year period (reviewed in Ref. 12 ) before the first 'oxicam' shown in panel e (CP-14304) was synthesized (see figure). The synthesis of this particular compound was a 'back burner' probe based on the intuition of the chemist. The introduction of a carboxamide function into the molecule proved to be a key factor in increasing anti-inflammatory activity and for increasing acidity. Structure–activity relationships (SARs) for several hundred analogous oxicam structures produced improved activity and safety, and, eventually, through a series of three development candidates (see figure parts c and e ), led to piroxicam as the agent that best met the project objectives. Extensive clinical trials confirmed the efficacy and safety of the new drug, leading to approvals and launches into major European markets in 1980, 18 years after the project was started. The drug provided around-the-clock symptom control for arthritis patients with just one 20-mg dose per day, leading to widespread acceptance by patients and making Feldene one of the most successful drugs in the 1980s. After 1992, major protective patents expired and generic brands of piroxicam dominated the market.
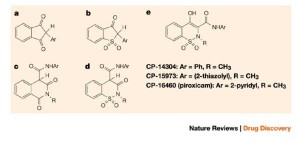
Box 2 | Discovery of ziprasidone (1984–2001)
Ziprasidone (Geodon; Pfizer) was launched in 2001 for the treatment of schizophrenia, a debilitating mental disease characterized by delusions, social withdrawal, suicidal behaviour and cognitive decline. The project that led to the discovery of ziprasidone relied primarily on disease-relevant animal models as had piroxicam ( Box 1 ), but, in addition, in vitro receptor-binding assays helped to find an agent that would lead to a significant advance over the already-available treatment.
The disease-relevant animal models for the ziprasidone discovery programme go back to the 1950s and the discovery of the first drug for schizophrenia (chlorpromazine), an anti-allergy drug that was serendipitously found to produce a calming effect in psychotic patients 19 . Paul Janssen, who had set up a medical research laboratory in 1953, studied the potential for discovering new antipsychotic drugs based on chlorpromazine by using it as a control drug in animal models designed to predict clinical activity. The models that Janssen developed relied on the ability of chlorpromazine to block the locomotor effects of stimulants such as amphetamine and apomorphine. Testing new agents that mimicked this activity of chlorpromazine led to his discovery of the first-generation antipsychotic drug haloperidol 20 . These models were still being used in the 1980s and therefore contributed to the discovery of ziprasidone.
As a supplementary approach to in vivo animal models as the primary screen, in vitro receptor-based pharmacology emerged in the 1980s and 1990s and came to dominate the field of antipsychotic drug research. This was based on the finding that agents such as haloperidol are effective antipsychotic drugs at the mechanistic level by virtue of their blockade of dopamine type 2 (D 2 ) receptors. In addition, clozapine — the first 'atypical' antipsychotic drug (so-called because it lacks the undesirable motor side effects of haloperidol and chlorpromazine, known as extrapyramidal symptoms (EPS)) — binds to both D 2 and 5-hydroxytryptamine type 2 (5-HT 2 ) receptors. The 5-HT 2 receptor for the neurotransmitter serotonin is thought to afford protection from EPSs that are caused by excessive D 2 -receptor blockade 21 , and this hypothesis initiated a search for an agent with a favourable (>10-fold) ratio of D 2 - to 5-HT 2 -receptor blockade 22 .
The search for ziprasidone began by considering the structure of naphthylpiperazine (compound 1 in the figure). Compound 1 was reported to be a potent ligand for serotonin receptors, including the 5-HT 2 receptor 23 . Combining compound 1 with the structure of dopamine, the natural ligand for the D 2 receptor, and then substituting the catechol with an oxindole as a surrogate produced the combined D 2 and 5-HT 2 antagonist compound 2 (see figure). Compound 2 seemed to be the perfect antipsychotic agent, at least in rats 24 . Further testing in monkeys, however, was disappointing, and attention switched to a new series derived from the 1,2-benzisothiazole group, which proved to have even more potent D 2 -receptor blockade while adding potent 5-HT 2 -receptor blockade that afforded the desired D 2 /5-HT 2 ratio 25 . Fine-tuning of the structure–activity relationship in this new series led from the prototype compound 3 to compound 4 (ziprasidone; see figure) 26 . Finally, the discovery programme confirmed the validity of the D 2 /5-HT 2 hypothesis using disease-relevant animal-model testing, which demonstrated efficacy without EPS liability. Following the 5-year-long discovery phase, another 9 years of clinical testing and 3 years to address regulatory requirements were needed before approval of ziprasidone was given by the FDA. Extensive clinical testing validated the discovery approach, and today hundreds of thousands of patient-days of use have demonstrated the efficacy and safety of ziprasidone as it continues to help patients afflicted with this lifelong, devastating disease.
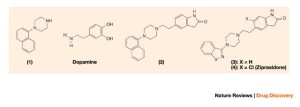
Box 3 | Discovery of imatinib mesylate
An illustration of the role of a defined molecular target coupled with structure-based drug design in drug discovery comes from the story of imatinib mesylate (Gleevec; Novartis), a selective tyrosine kinase inhibitor approved for the treatment of CHRONIC MYELOGENOUS LEUKAEMIA . The discovery of the oncogenes in the 1970s promised to aid the discovery of oncological drugs with reduced toxicity. In contrast to the cancer drugs in use then, which nonspecifically inhibited DNA synthesis and cell division, an oncogene inhibitor should be selectively toxic to cancer cells. Zimmerman and the Novartis group chose the tyrosine kinase BCR–ABL — which is created by a reciprocal chromosomal translocation that produces the BCR–ABL gene — as their target, as it is found only in leukaemic cells 27 . Inhibiting this molecularly defined target therefore reduces toxicity and maximizes the desired therapeutic effect. They chose compound 1 (see figure), an inhibitor of protein kinase C, as the starting point for the medicinal chemistry programme. Addition of the amide and methyl groups to the phenyl ring (see figure, compound 2) added the potency and selectivity needed for BCR–ABL inhibition, and addition of the piperazinylmethyl group (to generate imatinib, compound 3) was required for water solubility and oral bioavailability. Here is where the second advantage of a defined molecular target provides a crucial insight: when the X-ray crystal structure of imatinib bound to BCR–ABL was solved, it was found that the piperazine ring made significant contacts with the enzyme and was not just providing improved water solubility 28 . More importantly, these X-ray structure data provide insight into how mutations in the BCR–ABL gene produce an imatinib-resistant form of the enzyme, which offers the potential for designing new drugs to overcome this resistance.
Box 4 | In vitro tests: 'now' and 'then'
The following is a typical battery of tests for a modern drug discovery programme 'today'; those marked with an asterisk were also in use 'then'.
In vitro target
Selectivity assays
In vitro absorption, distribution, metabolism and elimination (ADME)
Microsomal stability
Hepatocyte stability
P450 substrate
P450 inhibitor
Permeability
Transporter efflux (for example, P-glycoprotein)
Protein binding
Physical properties
Rule-of-five
In silico ADME
* Secondary (behavioural, chronic)
* Ames test
Micronucleus test
hERG half-maximal inhibitory concentration (IC 50 )
P450 induction
Broad screening
* Others (depending on project)
Box 5 | Farnesyl transferase inhibitors
As one of the oncogenes characterized in the 1970s, RAS has been the target of numerous drug discovery efforts. Compounds that inhibit the enzyme farnesyl transferase (FTase) prevent the mutant form of RAS from causing tumour formation. A group at Merck has published extensively 29 on their FTase inhibitor programme, and examples from this programme are shown in the figure.
The table in the figure shows data for a set of compounds illustrating the criteria that the Merck group used to evaluate their compounds 30 . Compound 1 shows potent in vitro activity for the primary endpoint, farnesyl transferase (FTase) inhibition (IC 50 values are shown), as well as selectivity against geranyl geranyl transferase type I (GGTase), required for cell viability (IC 50 values are shown). Even though it shows good oral bioavailability (F) — 81% — it inhibits the hERG channel (the inflection point for binding to the hERG channel by radioligand displacement assay (hERG IP) = 440 nM) and causes QT PROLONGATION in the dog at a dose that is unacceptable. Macrocyclization to give compound 2 overcomes the problem with inhibition of hERG while maintaining in vitro potency, selectivity and oral bioavailability. In addition, X-ray crystal structure data of compound 2 bound to FTase explain how the enzyme accommodates this structural change and aids in further drug design. Increasing flexibility by saturating one of the rings of the naphthyl core in compound 2 to produce compound 3 and compound 4 considerably increases in vitro potency. Compound 3, however, is unfortunately very potent at hERG (80 nM), whereas compound 4 is cleared rapidly (rate of plasma clearance in the dog (CLp) = 7.3 ml per min per kg). So, even though it is the least potent compound in the set, compound 2 is the best choice for further structure–activity relationship development, primarily because of its pharmacokinetics and safety margin. This example illustrates why today's chemist more often prefers to begin with compounds that possess better pharmacokinetic and selectivity properties, and then to proceed to optimize potency for the primary in vitro end point. (Func 1, cell-based radiotracer assay for FTase inhibition; Func 2, cell-based assay for inhibition of FTase substrate derivatization, given in the absence and presence of human serum; N/A, not available; P450, IC 50 value for inhibition of human P450 3A4.)
R&D execs paint bleak picture of industry productivity: $1.4 bil. per NME. The Pink Sheet 6 (18 August 2003).
Demesmaeker, M. in Proc. 13th European Symp. Quantitative Structure-Activity Relationships: Rational Approaches to Drug Design (eds Hoeltje, H.-D. & Sippl, W.) 506–511 (Prous Science, Barcelona, 2000).
Google Scholar
Chanda, S. K. & Caldwell, J. S. Fulfilling the promise: drug discovery in the post-genomic era. Drug Discov. Today 8 , 168–174 (2003).
Article CAS Google Scholar
Reich, S. H. & Webber, S. E. Structure-based drug design (SBDD): every structure tells a story. Perspect. Drug Discov. Design 1 , 371–390 (1993).
Lin, J. H. & Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin. Pharmacokinet. 42 , 59–98 (2003).
Lin, J. et al. The role of absorption, distribution, metabolism, excretion and toxicity in drug discovery. Curr. Top. Med. Chem. 3 , 1125–1154 (2003). An excellent summary of the screening paradigm used in big pharma to evaluate new compounds for suitability as development candidates.
Article Google Scholar
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 46 , 3–26 (2001). A classic paper that outlines the use of physical properties to predict 'drug-likeness', used by most pharmaceutical companies to guide drug design.
Lipinski, C. A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 44 , 235–249 (2000).
Tseng, G. -N. IKr: The hERG channel. J. Mol. Cell. Cardiol. 33 , 835–849 (2001).
Taglialatela, M. et al. Cardiac ion channels and antihistamines: possible mechanisms of cardiotoxicity. Clin. Exp. Allergy 29 (Suppl. 3), 182–189 (1999).
Redfern, W. S. et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58 , 32–45 (2003).
Wiseman, E. H. & Lombardino, J. G. in Chronicles of Drug Discovery Ch. 8 (eds Bindra, J. S. & Lednicer, D.) 173–200 (J. Wiley and Sons, Inc., New York, 1982). An account of the discovery of the novel anti-inflammatory drug piroxicam.
Sapienza, A. M. & Lombardino, J. G. Recognizing, appreciating, and capturing tacit knowledge of R & D scientists. Drug Dev. Res. 57 , 51–57 (2002).
Mullen, R. Priming the pipeline. Chem. Eng. News 82 , 23–42 (2004).
Landers, P. Drug industry's big push into technology falls short. The Wall Street Journal A1 (24 February 2004).
Big Trouble for Big Pharma. The Economist 14 (16 December 2003).
Flynn, J. In two generations, drug research sees a big shift. The Wall Street Journal A1 (11 February 2004). An account of the careers of Leo Sternbach, inventor of Valium, and his son, Daniel, contrasting their experiences in drug discovery.
Lombardino, J. G. & Wiseman, E. H. Nonsteroidal Antiinflammatory Drugs — Mechanisms and Clinical Use Ch. 26 (eds Lewis, A. & Furst, D.) 487–506 (Marcel Dekker Inc., New York, 1987).
Delay, J., Deniker, P., Green, A. & Mordret, M. Excitor-motor syndromes provoked by ataratics. Presse Medicale (1893–1971), 65 , 1771–1774 (1957).
CAS PubMed Google Scholar
Granger, B. The discovery of haloperidol. Encephale , 25 , 59–66 (1999).
Hippius, H. A historical perspective of clozapine. J. Clin. Psychiatry 60 (Suppl. 12), 22–23 (1999). An account of the development history of the first atypical antipsychotic drug clozapine.
PubMed Google Scholar
Pere, J. J. Clinical psychopharmacology: the example of clozapine (Leponex). Encephale 21 (Spec. No. 3), 9–12 (1999).
Glennon, R. A., Slusher, R. M., Lyon, R. A., Titeler, M. & McKenney, J. D. 5-HT1 and 5-HT2: binding characteristics of some quipazine analogs. J. Med. Chem. 29 , 2375–2380 (1986).
Lowe, J. A. et al. 1-naphthylpiperazine derivatives as potential atypical antipsychotic agents. J. Med. Chem. 34 , 1860–1866 (1991).
Lowe, J. A. Atypical antipsychotics based on the D2/5-HT2 ratio hypothesis. Curr. Med. Chem. 1 , 50–60 (1994).
CAS Google Scholar
Howard, H. R. et al.. 3-benzisothiazolylpiperazine derivatives as potential atypical antipsychotic agents. J. Med. Chem. 39 , 143–148 (1996). An account of the discovery of the atypical antipsychotic drug ziprasidone.
Capdeville, R., Buchdunger, E., Zimmermann, J. & Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nature Rev. Drug Discov. 1 , 493–502 (2002). An account of the design and discovery of the anticancer drug imatinib mesylate (Gleevec; Novartis).
Schindler, T. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289 , 1938–1942 (2000).
Bell, I. M. et al. 3-aminopyrrolidinones farnesyltransferase inhibitors: design of macrocyclic compounds with improved pharmacokinetics and excellent cell potency. J. Med. Chem. 45 , 2388–2409 (2002). An account of a recent programme to discover novel anticancer agents that inhibit farnesyl transferase. Some of these data come from reference 30.
Bell, I. M. et al. Design and biological activity of ( S )-4-(5-{[1-(3-Chlorobenzyl)-2-oxopyrrolidin-3-ylamino]methyl}imidazol-1-ylmethyl)benzonitrile, a 3-aminopyrrolidinone farnesyltransferase inhibitor with excellent cell potency. J. Med. Chem. 44 , 2933–2949 (2001).
Download references
Author information
Authors and affiliations.
13 Laurel Hill Drive, Niantic, 06357, Connecticut, USA
Joseph G. Lombardino
Pfizer Global Research and Development, Pfizer Inc., MS 8220-4118, Eastern Point Road, Groton, 06340, Connecticut, USA
John A. Lowe III
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Joseph G. Lombardino or John A. Lowe III .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Related links
Alzheimer's disease
A chemical structure or series of structures that show activity and selectivity in a pharmacological or biochemically relevant screen.
The correlation of structural features with the activity of compounds in a given assay.
Synthetic technologies to generate compound libraries rather than single compounds.
(NME). A medication containing an active ingredient that has not been previously approved for marketing in the United States in any form.
A biologically active compound that exceeds a certain activity threshold in a given assay.
The study of the absorption, distribution, metabolism, excretion and interactions of a drug.
Members of the cytochrome P450 superfamily of haem proteins have a key role in the metabolism of drugs, and so understanding the roles of these enzymes is important for issues such as drug bioavailability and drug–drug interactions.
A haematological cancer characterized by excessive proliferation of cells of the myeloid lineage.
Sharing certain characteristics with other molecules that act as drugs. The set of characteristics — such as size, shape and solubility in water and organic solvents — varies depending on who is evaluating the molecules.
The fraction or percentage of an administered drug or other substance that becomes available in plasma or to the target tissue after administration.
The octanol/water partition coefficient is the ratio of the solubility of a compound in octanol to its solubility in water (also known as K ow ). The logarithm of this partition coefficient is called log P. It provides an estimate of the ability of the compound to pass through a cell membrane.
Human ether-a-go-go-related gene, the gene that encodes the α-subunit of the I Kr channel, a major determinant of human cardiac repolarization.
The QT interval is a measure of the total time of ventricular depolarization and repolarization. In recent years, several drugs have been withdrawn from the market because of unexpected reports of sudden cardiac death associated with prolongation of the QT interval. Blockade of the hERG channel has been linked to this effect.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lombardino, J., Lowe, J. The role of the medicinal chemist in drug discovery — then and now. Nat Rev Drug Discov 3 , 853–862 (2004). https://doi.org/10.1038/nrd1523
Download citation
Issue Date : October 2004
DOI : https://doi.org/10.1038/nrd1523
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
In-silico and in-vitro study reveals ziprasidone as a potential aromatase inhibitor against breast carcinoma.
- Ankita Sahu
- Shaban Ahmad
- Saurabh Verma
Scientific Reports (2023)
Predicting phytochemical diversity of medicinal and aromatic plants (MAPs) across eco-climatic zones and elevation in Uttarakhand using Generalized Additive Model
- Deepti Tiwari
- Pushpa Kewlani
- Veena Pande
Exploring the Potential of Compounds Isolated from Laranthus micranthus for the Treatment of Benign Prostatic Hyperplasia: Comprehensive Studies on Spectroscopic, Reactivity, and Biological Activity
- Richard U. Ukpanukpong
- Adindu E. Azubuike
- Hitler Louis
Chemistry Africa (2023)
Integrated data-driven and experimental approaches to accelerate lead optimization targeting SARS-CoV-2 main protease
- Rohith Anand Varikoti
- Katherine J. Schultz
- Neeraj Kumar
Journal of Computer-Aided Molecular Design (2023)
Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants
- YanYan Zhao
- Annalisa Cartabia
- Stéphane Declerck
Mycorrhiza (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Medicinal Chemistry Research is a journal focused on the publication of novel findings in drug design, drug discovery, and mechanisms of action of biologically active compounds.. Offers prompt publication of experimental achievements. Covers chemical biological relationships, especially on: structure-activity relationships, investigations of biochemical and pharmacological targets of drug ...
Medicinal chemistry articles from across Nature Portfolio. Medicinal chemistry deals with the design, optimization and development of chemical compounds for use as drugs. It is inherently a ...
The Journal of Medicinal Chemistry and ACS Medicinal Chemistry Letters are welcoming submissions to a Virtual Special Issue highlighting the importance of structural biology in drug discovery and therapeutic development. The submission deadline is January 31, 2025. Read the Editorial to learn more.
Medicinal Chemistry. Trust ACS to bring you experimental and theoretical studies across a broad range of medicinal chemistry, including compound design and optimization, biological evaluation, molecular mechanistic understanding of drug delivery and drug delivery systems, imaging agents, and pharmacology and translational science of both small ...
Docking-guided exploration of the anti-flt3 potential of isoindigo derivatives towards potential treatments of acute myeloid leukemia. Medicinal Chemistry Research is a journal focused on the publication of novel findings in drug design, drug discovery, and mechanisms of action of ...
Issue Publication Information. Journal of Medicinal Chemistry 2024, 67, 16, XXX-XXX (Article) Publication Date (Web): August 22, 2024. First Page. PDF. Check out the latest edition of the Journal of Medicinal Chemistry on ACS Publications, a trusted source for peer-reviewed journals.
Read the latest Research articles in Medicinal chemistry from Nature Chemistry. ... Medicinal chemistry articles within Nature ... One paper reports a method to screen for binders inside a living ...
All new research in RSC Medicinal Chemistry is published in the Research article format. Research articles have no page limits, although most articles fall between 4 and 10 journal pages (approximately 10-25 pages of double-spaced text). ... Research Articles encompass both full paper and communication styles. Where a communication style ...
Read the latest articles of Current Medicinal Chemistry at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature
Zafer Asım Kaplancıklı. 1 January 2023. Read latest issue. More opportunities to publish your research: Browse open Calls for Papers. Read the latest articles of Medicinal Chemistry at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Medicinal Chemistry Research is a journal for the prompt disclosure of novel experimental achievements in the many facets of drug design, drug discovery, and the elucidation of mechanisms of action of biologically active compounds. Articles are sought which emphasize research in chemical biological relationships, especially on: structure-activity relationships, investigations of biochemical ...
Research and review articles in medicinal chemistry and related drug discovery science. Editor-in-chief: Mike Waring Impact Factor: 4.1 Time to first decision (peer reviewed only): 30 days Submit your article Opens in new window Information and templates for authors
Medicinal chemistry is a fast-evolving interdisciplinary research area which aims to improve human life by developing drugs to combat diseases. Nature Communications interviewed three scientists ...
Read the latest articles of Current Topics in Medicinal Chemistry at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature
eISBN: 9781734427455. DOI: 10.1021/mc-2022-vol57. Joanne J. Bronson and Joachim Rudolph, Editors-In-Chief, the ACS Division of Medicinal Chemistry, and the American Chemical Society are pleased to announce the publication of 2022 Medicinal Chemistry Reviews, Volume 57 in the series. Become a member of the ACS Division of Medicinal Chemistry ...
PDF | Medicinal chemistry, a cornerstone of modern pharmaceutical science, plays a crucial role in the development of new therapeutic agents and the... | Find, read and cite all the research you ...
This Perspective highlights opportunities for expanding the synthetic toolbox of medicinal chemists, potentially enabling the more effective exploration of therapeutically relevant chemical space ...
Volume 31 January - December 2022. Issue 12 December 2022. Issue 11 November 2022. Issue 10 October 2022. Special issue of Medicinal Chemistry Research in honor of Professor Edmond J. LaVoie. Issue 9 September 2022. Issue 8 August 2022. Issue 7 July 2022. Special Issue in Honor of Prof. Laurence H. Hurley for His Many Contributions in Medicinal ...
In medicinal chemistry, thiophene derivatives are very important heterocycles exhibiting remarkable applications in different disciplines. In medicine, thiophene derivatives shows antimicrobial [6], analgesic and anti-inflammatory [7], antihypertensive [8], and antitumor activity [9] while they are also used as inhibitors of corrosion of metals ...
Medicinal chemistry is a fast-evolving interdisciplinary research area which aims to improve human life by developing drugs to combat diseases. interviewed. Nature Communications. three scientists ...
1. General information. Medicinal Chemistry Research is a journal for the prompt disclosure of novel experimental achievements in many facets of drug design, drug discovery, and the elucidation of mechanisms of action of biologically active compounds. Articles are sought which emphasize research in chemical biological relationships, especially ...
1. General information. Medicinal Chemistry Research is a journal for the prompt disclosure of novel experimental achievements in many facets of drug design, drug discovery, and the elucidation of mechanisms of action of biologically active compounds. Articles are sought which emphasize research in chemical biological relationships, especially ...
The present publication provides a comprehensive look at more than a decade (2010 to midyear of 2023) of medicinal chemistry research in India, focusing on contributions to medicinal chemistry and drug discovery from both Indian academia and industries. The work provides an overview of cutting-edge medicinal chemistry research along with the organic-transformation-based chemical research ...
Research and development (R&D ... These include a thorough knowledge of modern organic chemistry and medicinal chemistry, ... A classic paper that outlines the use of physical properties to ...