Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- How to Write a Strong Hypothesis | Steps & Examples

How to Write a Strong Hypothesis | Steps & Examples
Published on May 6, 2022 by Shona McCombes . Revised on November 20, 2023.
A hypothesis is a statement that can be tested by scientific research. If you want to test a relationship between two or more variables, you need to write hypotheses before you start your experiment or data collection .
Example: Hypothesis
Daily apple consumption leads to fewer doctor’s visits.
Table of contents
What is a hypothesis, developing a hypothesis (with example), hypothesis examples, other interesting articles, frequently asked questions about writing hypotheses.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess – it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Variables in hypotheses
Hypotheses propose a relationship between two or more types of variables .
- An independent variable is something the researcher changes or controls.
- A dependent variable is something the researcher observes and measures.
If there are any control variables , extraneous variables , or confounding variables , be sure to jot those down as you go to minimize the chances that research bias will affect your results.
In this example, the independent variable is exposure to the sun – the assumed cause . The dependent variable is the level of happiness – the assumed effect .
Prevent plagiarism. Run a free check.
Step 1. ask a question.
Writing a hypothesis begins with a research question that you want to answer. The question should be focused, specific, and researchable within the constraints of your project.
Step 2. Do some preliminary research
Your initial answer to the question should be based on what is already known about the topic. Look for theories and previous studies to help you form educated assumptions about what your research will find.
At this stage, you might construct a conceptual framework to ensure that you’re embarking on a relevant topic . This can also help you identify which variables you will study and what you think the relationships are between them. Sometimes, you’ll have to operationalize more complex constructs.
Step 3. Formulate your hypothesis
Now you should have some idea of what you expect to find. Write your initial answer to the question in a clear, concise sentence.
4. Refine your hypothesis
You need to make sure your hypothesis is specific and testable. There are various ways of phrasing a hypothesis, but all the terms you use should have clear definitions, and the hypothesis should contain:
- The relevant variables
- The specific group being studied
- The predicted outcome of the experiment or analysis
5. Phrase your hypothesis in three ways
To identify the variables, you can write a simple prediction in if…then form. The first part of the sentence states the independent variable and the second part states the dependent variable.
In academic research, hypotheses are more commonly phrased in terms of correlations or effects, where you directly state the predicted relationship between variables.
If you are comparing two groups, the hypothesis can state what difference you expect to find between them.
6. Write a null hypothesis
If your research involves statistical hypothesis testing , you will also have to write a null hypothesis . The null hypothesis is the default position that there is no association between the variables. The null hypothesis is written as H 0 , while the alternative hypothesis is H 1 or H a .
- H 0 : The number of lectures attended by first-year students has no effect on their final exam scores.
- H 1 : The number of lectures attended by first-year students has a positive effect on their final exam scores.
| Research question | Hypothesis | Null hypothesis |
|---|---|---|
| What are the health benefits of eating an apple a day? | Increasing apple consumption in over-60s will result in decreasing frequency of doctor’s visits. | Increasing apple consumption in over-60s will have no effect on frequency of doctor’s visits. |
| Which airlines have the most delays? | Low-cost airlines are more likely to have delays than premium airlines. | Low-cost and premium airlines are equally likely to have delays. |
| Can flexible work arrangements improve job satisfaction? | Employees who have flexible working hours will report greater job satisfaction than employees who work fixed hours. | There is no relationship between working hour flexibility and job satisfaction. |
| How effective is high school sex education at reducing teen pregnancies? | Teenagers who received sex education lessons throughout high school will have lower rates of unplanned pregnancy teenagers who did not receive any sex education. | High school sex education has no effect on teen pregnancy rates. |
| What effect does daily use of social media have on the attention span of under-16s? | There is a negative between time spent on social media and attention span in under-16s. | There is no relationship between social media use and attention span in under-16s. |
If you want to know more about the research process , methodology , research bias , or statistics , make sure to check out some of our other articles with explanations and examples.
- Sampling methods
- Simple random sampling
- Stratified sampling
- Cluster sampling
- Likert scales
- Reproducibility
Statistics
- Null hypothesis
- Statistical power
- Probability distribution
- Effect size
- Poisson distribution
Research bias
- Optimism bias
- Cognitive bias
- Implicit bias
- Hawthorne effect
- Anchoring bias
- Explicit bias
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

A hypothesis is not just a guess — it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations and statistical analysis of data).
Null and alternative hypotheses are used in statistical hypothesis testing . The null hypothesis of a test always predicts no effect or no relationship between variables, while the alternative hypothesis states your research prediction of an effect or relationship.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, November 20). How to Write a Strong Hypothesis | Steps & Examples. Scribbr. Retrieved August 5, 2024, from https://www.scribbr.com/methodology/hypothesis/
Is this article helpful?
Shona McCombes
Other students also liked, construct validity | definition, types, & examples, what is a conceptual framework | tips & examples, operationalization | a guide with examples, pros & cons, what is your plagiarism score.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Hypothesis Examples

A hypothesis is a prediction of the outcome of a test. It forms the basis for designing an experiment in the scientific method . A good hypothesis is testable, meaning it makes a prediction you can check with observation or experimentation. Here are different hypothesis examples.
Null Hypothesis Examples
The null hypothesis (H 0 ) is also known as the zero-difference or no-difference hypothesis. It predicts that changing one variable ( independent variable ) will have no effect on the variable being measured ( dependent variable ). Here are null hypothesis examples:
- Plant growth is unaffected by temperature.
- If you increase temperature, then solubility of salt will increase.
- Incidence of skin cancer is unrelated to ultraviolet light exposure.
- All brands of light bulb last equally long.
- Cats have no preference for the color of cat food.
- All daisies have the same number of petals.
Sometimes the null hypothesis shows there is a suspected correlation between two variables. For example, if you think plant growth is affected by temperature, you state the null hypothesis: “Plant growth is not affected by temperature.” Why do you do this, rather than say “If you change temperature, plant growth will be affected”? The answer is because it’s easier applying a statistical test that shows, with a high level of confidence, a null hypothesis is correct or incorrect.
Research Hypothesis Examples
A research hypothesis (H 1 ) is a type of hypothesis used to design an experiment. This type of hypothesis is often written as an if-then statement because it’s easy identifying the independent and dependent variables and seeing how one affects the other. If-then statements explore cause and effect. In other cases, the hypothesis shows a correlation between two variables. Here are some research hypothesis examples:
- If you leave the lights on, then it takes longer for people to fall asleep.
- If you refrigerate apples, they last longer before going bad.
- If you keep the curtains closed, then you need less electricity to heat or cool the house (the electric bill is lower).
- If you leave a bucket of water uncovered, then it evaporates more quickly.
- Goldfish lose their color if they are not exposed to light.
- Workers who take vacations are more productive than those who never take time off.
Is It Okay to Disprove a Hypothesis?
Yes! You may even choose to write your hypothesis in such a way that it can be disproved because it’s easier to prove a statement is wrong than to prove it is right. In other cases, if your prediction is incorrect, that doesn’t mean the science is bad. Revising a hypothesis is common. It demonstrates you learned something you did not know before you conducted the experiment.
Test yourself with a Scientific Method Quiz .
- Mellenbergh, G.J. (2008). Chapter 8: Research designs: Testing of research hypotheses. In H.J. Adèr & G.J. Mellenbergh (eds.), Advising on Research Methods: A Consultant’s Companion . Huizen, The Netherlands: Johannes van Kessel Publishing.
- Popper, Karl R. (1959). The Logic of Scientific Discovery . Hutchinson & Co. ISBN 3-1614-8410-X.
- Schick, Theodore; Vaughn, Lewis (2002). How to think about weird things: critical thinking for a New Age . Boston: McGraw-Hill Higher Education. ISBN 0-7674-2048-9.
- Tobi, Hilde; Kampen, Jarl K. (2018). “Research design: the methodology for interdisciplinary research framework”. Quality & Quantity . 52 (3): 1209–1225. doi: 10.1007/s11135-017-0513-8
Related Posts

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- When did science begin?
- Where was science invented?

scientific hypothesis
Our editors will review what you’ve submitted and determine whether to revise the article.
- National Center for Biotechnology Information - PubMed Central - On the scope of scientific hypotheses
- LiveScience - What is a scientific hypothesis?
- The Royal Society - Open Science - On the scope of scientific hypotheses

scientific hypothesis , an idea that proposes a tentative explanation about a phenomenon or a narrow set of phenomena observed in the natural world. The two primary features of a scientific hypothesis are falsifiability and testability, which are reflected in an “If…then” statement summarizing the idea and in the ability to be supported or refuted through observation and experimentation. The notion of the scientific hypothesis as both falsifiable and testable was advanced in the mid-20th century by Austrian-born British philosopher Karl Popper .
The formulation and testing of a hypothesis is part of the scientific method , the approach scientists use when attempting to understand and test ideas about natural phenomena. The generation of a hypothesis frequently is described as a creative process and is based on existing scientific knowledge, intuition , or experience. Therefore, although scientific hypotheses commonly are described as educated guesses, they actually are more informed than a guess. In addition, scientists generally strive to develop simple hypotheses, since these are easier to test relative to hypotheses that involve many different variables and potential outcomes. Such complex hypotheses may be developed as scientific models ( see scientific modeling ).
Depending on the results of scientific evaluation, a hypothesis typically is either rejected as false or accepted as true. However, because a hypothesis inherently is falsifiable, even hypotheses supported by scientific evidence and accepted as true are susceptible to rejection later, when new evidence has become available. In some instances, rather than rejecting a hypothesis because it has been falsified by new evidence, scientists simply adapt the existing idea to accommodate the new information. In this sense a hypothesis is never incorrect but only incomplete.
The investigation of scientific hypotheses is an important component in the development of scientific theory . Hence, hypotheses differ fundamentally from theories; whereas the former is a specific tentative explanation and serves as the main tool by which scientists gather data, the latter is a broad general explanation that incorporates data from many different scientific investigations undertaken to explore hypotheses.
Countless hypotheses have been developed and tested throughout the history of science . Several examples include the idea that living organisms develop from nonliving matter, which formed the basis of spontaneous generation , a hypothesis that ultimately was disproved (first in 1668, with the experiments of Italian physician Francesco Redi , and later in 1859, with the experiments of French chemist and microbiologist Louis Pasteur ); the concept proposed in the late 19th century that microorganisms cause certain diseases (now known as germ theory ); and the notion that oceanic crust forms along submarine mountain zones and spreads laterally away from them ( seafloor spreading hypothesis ).
What Is a Hypothesis? (Science)
If...,Then...
Angela Lumsden/Getty Images
- Scientific Method
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A hypothesis (plural hypotheses) is a proposed explanation for an observation. The definition depends on the subject.
In science, a hypothesis is part of the scientific method. It is a prediction or explanation that is tested by an experiment. Observations and experiments may disprove a scientific hypothesis, but can never entirely prove one.
In the study of logic, a hypothesis is an if-then proposition, typically written in the form, "If X , then Y ."
In common usage, a hypothesis is simply a proposed explanation or prediction, which may or may not be tested.
Writing a Hypothesis
Most scientific hypotheses are proposed in the if-then format because it's easy to design an experiment to see whether or not a cause and effect relationship exists between the independent variable and the dependent variable . The hypothesis is written as a prediction of the outcome of the experiment.
Null Hypothesis and Alternative Hypothesis
Statistically, it's easier to show there is no relationship between two variables than to support their connection. So, scientists often propose the null hypothesis . The null hypothesis assumes changing the independent variable will have no effect on the dependent variable.
In contrast, the alternative hypothesis suggests changing the independent variable will have an effect on the dependent variable. Designing an experiment to test this hypothesis can be trickier because there are many ways to state an alternative hypothesis.
For example, consider a possible relationship between getting a good night's sleep and getting good grades. The null hypothesis might be stated: "The number of hours of sleep students get is unrelated to their grades" or "There is no correlation between hours of sleep and grades."
An experiment to test this hypothesis might involve collecting data, recording average hours of sleep for each student and grades. If a student who gets eight hours of sleep generally does better than students who get four hours of sleep or 10 hours of sleep, the hypothesis might be rejected.
But the alternative hypothesis is harder to propose and test. The most general statement would be: "The amount of sleep students get affects their grades." The hypothesis might also be stated as "If you get more sleep, your grades will improve" or "Students who get nine hours of sleep have better grades than those who get more or less sleep."
In an experiment, you can collect the same data, but the statistical analysis is less likely to give you a high confidence limit.
Usually, a scientist starts out with the null hypothesis. From there, it may be possible to propose and test an alternative hypothesis, to narrow down the relationship between the variables.
Example of a Hypothesis
Examples of a hypothesis include:
- If you drop a rock and a feather, (then) they will fall at the same rate.
- Plants need sunlight in order to live. (if sunlight, then life)
- Eating sugar gives you energy. (if sugar, then energy)
- White, Jay D. Research in Public Administration . Conn., 1998.
- Schick, Theodore, and Lewis Vaughn. How to Think about Weird Things: Critical Thinking for a New Age . McGraw-Hill Higher Education, 2002.
- Scientific Method Flow Chart
- What Are the Elements of a Good Hypothesis?
- Six Steps of the Scientific Method
- What Are Examples of a Hypothesis?
- What Is a Testable Hypothesis?
- Null Hypothesis Examples
- Scientific Hypothesis Examples
- Scientific Variable
- Scientific Method Vocabulary Terms
- Understanding Simple vs Controlled Experiments
- What Is a Controlled Experiment?
- What Is an Experimental Constant?
- What Is the Difference Between a Control Variable and Control Group?
- DRY MIX Experiment Variables Acronym
- Random Error vs. Systematic Error
- The Role of a Controlled Variable in an Experiment
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- How to Write a Strong Hypothesis | Guide & Examples
How to Write a Strong Hypothesis | Guide & Examples
Published on 6 May 2022 by Shona McCombes .
A hypothesis is a statement that can be tested by scientific research. If you want to test a relationship between two or more variables, you need to write hypotheses before you start your experiment or data collection.
Table of contents
What is a hypothesis, developing a hypothesis (with example), hypothesis examples, frequently asked questions about writing hypotheses.
A hypothesis states your predictions about what your research will find. It is a tentative answer to your research question that has not yet been tested. For some research projects, you might have to write several hypotheses that address different aspects of your research question.
A hypothesis is not just a guess – it should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations, and statistical analysis of data).
Variables in hypotheses
Hypotheses propose a relationship between two or more variables . An independent variable is something the researcher changes or controls. A dependent variable is something the researcher observes and measures.
In this example, the independent variable is exposure to the sun – the assumed cause . The dependent variable is the level of happiness – the assumed effect .
Prevent plagiarism, run a free check.
Step 1: ask a question.
Writing a hypothesis begins with a research question that you want to answer. The question should be focused, specific, and researchable within the constraints of your project.
Step 2: Do some preliminary research
Your initial answer to the question should be based on what is already known about the topic. Look for theories and previous studies to help you form educated assumptions about what your research will find.
At this stage, you might construct a conceptual framework to identify which variables you will study and what you think the relationships are between them. Sometimes, you’ll have to operationalise more complex constructs.
Step 3: Formulate your hypothesis
Now you should have some idea of what you expect to find. Write your initial answer to the question in a clear, concise sentence.
Step 4: Refine your hypothesis
You need to make sure your hypothesis is specific and testable. There are various ways of phrasing a hypothesis, but all the terms you use should have clear definitions, and the hypothesis should contain:
- The relevant variables
- The specific group being studied
- The predicted outcome of the experiment or analysis
Step 5: Phrase your hypothesis in three ways
To identify the variables, you can write a simple prediction in if … then form. The first part of the sentence states the independent variable and the second part states the dependent variable.
In academic research, hypotheses are more commonly phrased in terms of correlations or effects, where you directly state the predicted relationship between variables.
If you are comparing two groups, the hypothesis can state what difference you expect to find between them.
Step 6. Write a null hypothesis
If your research involves statistical hypothesis testing , you will also have to write a null hypothesis. The null hypothesis is the default position that there is no association between the variables. The null hypothesis is written as H 0 , while the alternative hypothesis is H 1 or H a .
| Research question | Hypothesis | Null hypothesis |
|---|---|---|
| What are the health benefits of eating an apple a day? | Increasing apple consumption in over-60s will result in decreasing frequency of doctor’s visits. | Increasing apple consumption in over-60s will have no effect on frequency of doctor’s visits. |
| Which airlines have the most delays? | Low-cost airlines are more likely to have delays than premium airlines. | Low-cost and premium airlines are equally likely to have delays. |
| Can flexible work arrangements improve job satisfaction? | Employees who have flexible working hours will report greater job satisfaction than employees who work fixed hours. | There is no relationship between working hour flexibility and job satisfaction. |
| How effective is secondary school sex education at reducing teen pregnancies? | Teenagers who received sex education lessons throughout secondary school will have lower rates of unplanned pregnancy than teenagers who did not receive any sex education. | Secondary school sex education has no effect on teen pregnancy rates. |
| What effect does daily use of social media have on the attention span of under-16s? | There is a negative correlation between time spent on social media and attention span in under-16s. | There is no relationship between social media use and attention span in under-16s. |
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
A hypothesis is not just a guess. It should be based on existing theories and knowledge. It also has to be testable, which means you can support or refute it through scientific research methods (such as experiments, observations, and statistical analysis of data).
A research hypothesis is your proposed answer to your research question. The research hypothesis usually includes an explanation (‘ x affects y because …’).
A statistical hypothesis, on the other hand, is a mathematical statement about a population parameter. Statistical hypotheses always come in pairs: the null and alternative hypotheses. In a well-designed study , the statistical hypotheses correspond logically to the research hypothesis.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
McCombes, S. (2022, May 06). How to Write a Strong Hypothesis | Guide & Examples. Scribbr. Retrieved 5 August 2024, from https://www.scribbr.co.uk/research-methods/hypothesis-writing/
Is this article helpful?
Shona McCombes
Other students also liked, operationalisation | a guide with examples, pros & cons, what is a conceptual framework | tips & examples, a quick guide to experimental design | 5 steps & examples.
What is a scientific hypothesis?
It's the initial building block in the scientific method.

Hypothesis basics
What makes a hypothesis testable.
- Types of hypotheses
- Hypothesis versus theory
Additional resources
Bibliography.
A scientific hypothesis is a tentative, testable explanation for a phenomenon in the natural world. It's the initial building block in the scientific method . Many describe it as an "educated guess" based on prior knowledge and observation. While this is true, a hypothesis is more informed than a guess. While an "educated guess" suggests a random prediction based on a person's expertise, developing a hypothesis requires active observation and background research.
The basic idea of a hypothesis is that there is no predetermined outcome. For a solution to be termed a scientific hypothesis, it has to be an idea that can be supported or refuted through carefully crafted experimentation or observation. This concept, called falsifiability and testability, was advanced in the mid-20th century by Austrian-British philosopher Karl Popper in his famous book "The Logic of Scientific Discovery" (Routledge, 1959).
A key function of a hypothesis is to derive predictions about the results of future experiments and then perform those experiments to see whether they support the predictions.
A hypothesis is usually written in the form of an if-then statement, which gives a possibility (if) and explains what may happen because of the possibility (then). The statement could also include "may," according to California State University, Bakersfield .
Here are some examples of hypothesis statements:
- If garlic repels fleas, then a dog that is given garlic every day will not get fleas.
- If sugar causes cavities, then people who eat a lot of candy may be more prone to cavities.
- If ultraviolet light can damage the eyes, then maybe this light can cause blindness.
A useful hypothesis should be testable and falsifiable. That means that it should be possible to prove it wrong. A theory that can't be proved wrong is nonscientific, according to Karl Popper's 1963 book " Conjectures and Refutations ."
An example of an untestable statement is, "Dogs are better than cats." That's because the definition of "better" is vague and subjective. However, an untestable statement can be reworded to make it testable. For example, the previous statement could be changed to this: "Owning a dog is associated with higher levels of physical fitness than owning a cat." With this statement, the researcher can take measures of physical fitness from dog and cat owners and compare the two.
Types of scientific hypotheses

In an experiment, researchers generally state their hypotheses in two ways. The null hypothesis predicts that there will be no relationship between the variables tested, or no difference between the experimental groups. The alternative hypothesis predicts the opposite: that there will be a difference between the experimental groups. This is usually the hypothesis scientists are most interested in, according to the University of Miami .
For example, a null hypothesis might state, "There will be no difference in the rate of muscle growth between people who take a protein supplement and people who don't." The alternative hypothesis would state, "There will be a difference in the rate of muscle growth between people who take a protein supplement and people who don't."
If the results of the experiment show a relationship between the variables, then the null hypothesis has been rejected in favor of the alternative hypothesis, according to the book " Research Methods in Psychology " (BCcampus, 2015).
There are other ways to describe an alternative hypothesis. The alternative hypothesis above does not specify a direction of the effect, only that there will be a difference between the two groups. That type of prediction is called a two-tailed hypothesis. If a hypothesis specifies a certain direction — for example, that people who take a protein supplement will gain more muscle than people who don't — it is called a one-tailed hypothesis, according to William M. K. Trochim , a professor of Policy Analysis and Management at Cornell University.
Sometimes, errors take place during an experiment. These errors can happen in one of two ways. A type I error is when the null hypothesis is rejected when it is true. This is also known as a false positive. A type II error occurs when the null hypothesis is not rejected when it is false. This is also known as a false negative, according to the University of California, Berkeley .
A hypothesis can be rejected or modified, but it can never be proved correct 100% of the time. For example, a scientist can form a hypothesis stating that if a certain type of tomato has a gene for red pigment, that type of tomato will be red. During research, the scientist then finds that each tomato of this type is red. Though the findings confirm the hypothesis, there may be a tomato of that type somewhere in the world that isn't red. Thus, the hypothesis is true, but it may not be true 100% of the time.
Scientific theory vs. scientific hypothesis
The best hypotheses are simple. They deal with a relatively narrow set of phenomena. But theories are broader; they generally combine multiple hypotheses into a general explanation for a wide range of phenomena, according to the University of California, Berkeley . For example, a hypothesis might state, "If animals adapt to suit their environments, then birds that live on islands with lots of seeds to eat will have differently shaped beaks than birds that live on islands with lots of insects to eat." After testing many hypotheses like these, Charles Darwin formulated an overarching theory: the theory of evolution by natural selection.
"Theories are the ways that we make sense of what we observe in the natural world," Tanner said. "Theories are structures of ideas that explain and interpret facts."
- Read more about writing a hypothesis, from the American Medical Writers Association.
- Find out why a hypothesis isn't always necessary in science, from The American Biology Teacher.
- Learn about null and alternative hypotheses, from Prof. Essa on YouTube .
Encyclopedia Britannica. Scientific Hypothesis. Jan. 13, 2022. https://www.britannica.com/science/scientific-hypothesis
Karl Popper, "The Logic of Scientific Discovery," Routledge, 1959.
California State University, Bakersfield, "Formatting a testable hypothesis." https://www.csub.edu/~ddodenhoff/Bio100/Bio100sp04/formattingahypothesis.htm
Karl Popper, "Conjectures and Refutations," Routledge, 1963.
Price, P., Jhangiani, R., & Chiang, I., "Research Methods of Psychology — 2nd Canadian Edition," BCcampus, 2015.
University of Miami, "The Scientific Method" http://www.bio.miami.edu/dana/161/evolution/161app1_scimethod.pdf
William M.K. Trochim, "Research Methods Knowledge Base," https://conjointly.com/kb/hypotheses-explained/
University of California, Berkeley, "Multiple Hypothesis Testing and False Discovery Rate" https://www.stat.berkeley.edu/~hhuang/STAT141/Lecture-FDR.pdf
University of California, Berkeley, "Science at multiple levels" https://undsci.berkeley.edu/article/0_0_0/howscienceworks_19

Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Why scientists are blown away by 'Twister' and 'Twisters'
3 remarkable trees: A living fossil, a deadly canopy, and the world's biggest seeds that were once mounted in gold by royals
The Perseid meteor shower is about to peak. Here's when to see the most 'shooting stars'.
Most Popular
- 2 No, NASA hasn't warned of an impending asteroid strike in 2038. Here's what really happened.
- 3 Milky Way's black hole 'exhaust vent' discovered in eerie X-ray observations
- 4 NASA offers SpaceX $843 million to destroy the International Space Station
- 5 Which continent has the most animal species?
- 2 2,000 years ago, a bridge in Switzerland collapsed on top of Celtic sacrifice victims, new study suggests
- 3 Self-healing 'living skin' can make robots more humanlike — and it looks just as creepy as you'd expect
- 4 Tasselled wobbegong: The master of disguise that can eat a shark almost as big as itself
- 5 This robot could leap higher than the Statue of Liberty — if we ever build it properly
- Scientific Methods
What is Hypothesis?
We have heard of many hypotheses which have led to great inventions in science. Assumptions that are made on the basis of some evidence are known as hypotheses. In this article, let us learn in detail about the hypothesis and the type of hypothesis with examples.

A hypothesis is an assumption that is made based on some evidence. This is the initial point of any investigation that translates the research questions into predictions. It includes components like variables, population and the relation between the variables. A research hypothesis is a hypothesis that is used to test the relationship between two or more variables.
Characteristics of Hypothesis
Following are the characteristics of the hypothesis:
- The hypothesis should be clear and precise to consider it to be reliable.
- If the hypothesis is a relational hypothesis, then it should be stating the relationship between variables.
- The hypothesis must be specific and should have scope for conducting more tests.
- The way of explanation of the hypothesis must be very simple and it should also be understood that the simplicity of the hypothesis is not related to its significance.
Sources of Hypothesis
Following are the sources of hypothesis:
- The resemblance between the phenomenon.
- Observations from past studies, present-day experiences and from the competitors.
- Scientific theories.
- General patterns that influence the thinking process of people.
Types of Hypothesis
There are six forms of hypothesis and they are:
- Simple hypothesis
- Complex hypothesis
- Directional hypothesis
- Non-directional hypothesis
- Null hypothesis
- Associative and casual hypothesis
Simple Hypothesis
It shows a relationship between one dependent variable and a single independent variable. For example – If you eat more vegetables, you will lose weight faster. Here, eating more vegetables is an independent variable, while losing weight is the dependent variable.
Complex Hypothesis
It shows the relationship between two or more dependent variables and two or more independent variables. Eating more vegetables and fruits leads to weight loss, glowing skin, and reduces the risk of many diseases such as heart disease.
Directional Hypothesis
It shows how a researcher is intellectual and committed to a particular outcome. The relationship between the variables can also predict its nature. For example- children aged four years eating proper food over a five-year period are having higher IQ levels than children not having a proper meal. This shows the effect and direction of the effect.
Non-directional Hypothesis
It is used when there is no theory involved. It is a statement that a relationship exists between two variables, without predicting the exact nature (direction) of the relationship.
Null Hypothesis
It provides a statement which is contrary to the hypothesis. It’s a negative statement, and there is no relationship between independent and dependent variables. The symbol is denoted by “H O ”.
Associative and Causal Hypothesis
Associative hypothesis occurs when there is a change in one variable resulting in a change in the other variable. Whereas, the causal hypothesis proposes a cause and effect interaction between two or more variables.
Examples of Hypothesis
Following are the examples of hypotheses based on their types:
- Consumption of sugary drinks every day leads to obesity is an example of a simple hypothesis.
- All lilies have the same number of petals is an example of a null hypothesis.
- If a person gets 7 hours of sleep, then he will feel less fatigue than if he sleeps less. It is an example of a directional hypothesis.
Functions of Hypothesis
Following are the functions performed by the hypothesis:
- Hypothesis helps in making an observation and experiments possible.
- It becomes the start point for the investigation.
- Hypothesis helps in verifying the observations.
- It helps in directing the inquiries in the right direction.
How will Hypothesis help in the Scientific Method?
Researchers use hypotheses to put down their thoughts directing how the experiment would take place. Following are the steps that are involved in the scientific method:
- Formation of question
- Doing background research
- Creation of hypothesis
- Designing an experiment
- Collection of data
- Result analysis
- Summarizing the experiment
- Communicating the results
Frequently Asked Questions – FAQs
What is hypothesis.
A hypothesis is an assumption made based on some evidence.
Give an example of simple hypothesis?
What are the types of hypothesis.
Types of hypothesis are:
- Associative and Casual hypothesis
State true or false: Hypothesis is the initial point of any investigation that translates the research questions into a prediction.
Define complex hypothesis..
A complex hypothesis shows the relationship between two or more dependent variables and two or more independent variables.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
| PHYSICS Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
1.2 The Scientific Methods
Section learning objectives.
By the end of this section, you will be able to do the following:
- Explain how the methods of science are used to make scientific discoveries
- Define a scientific model and describe examples of physical and mathematical models used in physics
- Compare and contrast hypothesis, theory, and law
Teacher Support
The learning objectives in this section will help your students master the following standards:
- (A) know the definition of science and understand that it has limitations, as specified in subsection (b)(2) of this section;
- (B) know that scientific hypotheses are tentative and testable statements that must be capable of being supported or not supported by observational evidence. Hypotheses of durable explanatory power which have been tested over a wide variety of conditions are incorporated into theories;
- (C) know that scientific theories are based on natural and physical phenomena and are capable of being tested by multiple independent researchers. Unlike hypotheses, scientific theories are well-established and highly-reliable explanations, but may be subject to change as new areas of science and new technologies are developed;
- (D) distinguish between scientific hypotheses and scientific theories.
Section Key Terms
| experiment | hypothesis | model | observation | principle |
| scientific law | scientific methods | theory | universal |
[OL] Pre-assessment for this section could involve students sharing or writing down an anecdote about when they used the methods of science. Then, students could label their thought processes in their anecdote with the appropriate scientific methods. The class could also discuss their definitions of theory and law, both outside and within the context of science.
[OL] It should be noted and possibly mentioned that a scientist , as mentioned in this section, does not necessarily mean a trained scientist. It could be anyone using methods of science.
Scientific Methods
Scientists often plan and carry out investigations to answer questions about the universe around us. These investigations may lead to natural laws. Such laws are intrinsic to the universe, meaning that humans did not create them and cannot change them. We can only discover and understand them. Their discovery is a very human endeavor, with all the elements of mystery, imagination, struggle, triumph, and disappointment inherent in any creative effort. The cornerstone of discovering natural laws is observation. Science must describe the universe as it is, not as we imagine or wish it to be.
We all are curious to some extent. We look around, make generalizations, and try to understand what we see. For example, we look up and wonder whether one type of cloud signals an oncoming storm. As we become serious about exploring nature, we become more organized and formal in collecting and analyzing data. We attempt greater precision, perform controlled experiments (if we can), and write down ideas about how data may be organized. We then formulate models, theories, and laws based on the data we have collected, and communicate those results with others. This, in a nutshell, describes the scientific method that scientists employ to decide scientific issues on the basis of evidence from observation and experiment.
An investigation often begins with a scientist making an observation . The scientist observes a pattern or trend within the natural world. Observation may generate questions that the scientist wishes to answer. Next, the scientist may perform some research about the topic and devise a hypothesis . A hypothesis is a testable statement that describes how something in the natural world works. In essence, a hypothesis is an educated guess that explains something about an observation.
[OL] An educated guess is used throughout this section in describing a hypothesis to combat the tendency to think of a theory as an educated guess.
Scientists may test the hypothesis by performing an experiment . During an experiment, the scientist collects data that will help them learn about the phenomenon they are studying. Then the scientists analyze the results of the experiment (that is, the data), often using statistical, mathematical, and/or graphical methods. From the data analysis, they draw conclusions. They may conclude that their experiment either supports or rejects their hypothesis. If the hypothesis is supported, the scientist usually goes on to test another hypothesis related to the first. If their hypothesis is rejected, they will often then test a new and different hypothesis in their effort to learn more about whatever they are studying.
Scientific processes can be applied to many situations. Let’s say that you try to turn on your car, but it will not start. You have just made an observation! You ask yourself, "Why won’t my car start?" You can now use scientific processes to answer this question. First, you generate a hypothesis such as, "The car won’t start because it has no gasoline in the gas tank." To test this hypothesis, you put gasoline in the car and try to start it again. If the car starts, then your hypothesis is supported by the experiment. If the car does not start, then your hypothesis is rejected. You will then need to think up a new hypothesis to test such as, "My car won’t start because the fuel pump is broken." Hopefully, your investigations lead you to discover why the car won’t start and enable you to fix it.
A model is a representation of something that is often too difficult (or impossible) to study directly. Models can take the form of physical models, equations, computer programs, or simulations—computer graphics/animations. Models are tools that are especially useful in modern physics because they let us visualize phenomena that we normally cannot observe with our senses, such as very small objects or objects that move at high speeds. For example, we can understand the structure of an atom using models, without seeing an atom with our own eyes. Although images of single atoms are now possible, these images are extremely difficult to achieve and are only possible due to the success of our models. The existence of these images is a consequence rather than a source of our understanding of atoms. Models are always approximate, so they are simpler to consider than the real situation; the more complete a model is, the more complicated it must be. Models put the intangible or the extremely complex into human terms that we can visualize, discuss, and hypothesize about.
Scientific models are constructed based on the results of previous experiments. Even still, models often only describe a phenomenon partially or in a few limited situations. Some phenomena are so complex that they may be impossible to model them in their entirety, even using computers. An example is the electron cloud model of the atom in which electrons are moving around the atom’s center in distinct clouds ( Figure 1.12 ), that represent the likelihood of finding an electron in different places. This model helps us to visualize the structure of an atom. However, it does not show us exactly where an electron will be within its cloud at any one particular time.
As mentioned previously, physicists use a variety of models including equations, physical models, computer simulations, etc. For example, three-dimensional models are often commonly used in chemistry and physics to model molecules. Properties other than appearance or location are usually modelled using mathematics, where functions are used to show how these properties relate to one another. Processes such as the formation of a star or the planets, can also be modelled using computer simulations. Once a simulation is correctly programmed based on actual experimental data, the simulation can allow us to view processes that happened in the past or happen too quickly or slowly for us to observe directly. In addition, scientists can also run virtual experiments using computer-based models. In a model of planet formation, for example, the scientist could alter the amount or type of rocks present in space and see how it affects planet formation.
Scientists use models and experimental results to construct explanations of observations or design solutions to problems. For example, one way to make a car more fuel efficient is to reduce the friction or drag caused by air flowing around the moving car. This can be done by designing the body shape of the car to be more aerodynamic, such as by using rounded corners instead of sharp ones. Engineers can then construct physical models of the car body, place them in a wind tunnel, and examine the flow of air around the model. This can also be done mathematically in a computer simulation. The air flow pattern can be analyzed for regions smooth air flow and for eddies that indicate drag. The model of the car body may have to be altered slightly to produce the smoothest pattern of air flow (i.e., the least drag). The pattern with the least drag may be the solution to increasing fuel efficiency of the car. This solution might then be incorporated into the car design.
Using Models and the Scientific Processes
Be sure to secure loose items before opening the window or door.
In this activity, you will learn about scientific models by making a model of how air flows through your classroom or a room in your house.
- One room with at least one window or door that can be opened
- Work with a group of four, as directed by your teacher. Close all of the windows and doors in the room you are working in. Your teacher may assign you a specific window or door to study.
- Before opening any windows or doors, draw a to-scale diagram of your room. First, measure the length and width of your room using the tape measure. Then, transform the measurement using a scale that could fit on your paper, such as 5 centimeters = 1 meter.
- Your teacher will assign you a specific window or door to study air flow. On your diagram, add arrows showing your hypothesis (before opening any windows or doors) of how air will flow through the room when your assigned window or door is opened. Use pencil so that you can easily make changes to your diagram.
- On your diagram, mark four locations where you would like to test air flow in your room. To test for airflow, hold a strip of single ply tissue paper between the thumb and index finger. Note the direction that the paper moves when exposed to the airflow. Then, for each location, predict which way the paper will move if your air flow diagram is correct.
- Now, each member of your group will stand in one of the four selected areas. Each member will test the airflow Agree upon an approximate height at which everyone will hold their papers.
- When you teacher tells you to, open your assigned window and/or door. Each person should note the direction that their paper points immediately after the window or door was opened. Record your results on your diagram.
- Did the airflow test data support or refute the hypothetical model of air flow shown in your diagram? Why or why not? Correct your model based on your experimental evidence.
- With your group, discuss how accurate your model is. What limitations did it have? Write down the limitations that your group agreed upon.
- Yes, you could use your model to predict air flow through a new window. The earlier experiment of air flow would help you model the system more accurately.
- Yes, you could use your model to predict air flow through a new window. The earlier experiment of air flow is not useful for modeling the new system.
- No, you cannot model a system to predict the air flow through a new window. The earlier experiment of air flow would help you model the system more accurately.
- No, you cannot model a system to predict the air flow through a new window. The earlier experiment of air flow is not useful for modeling the new system.
This Snap Lab! has students construct a model of how air flows in their classroom. Each group of four students will create a model of air flow in their classroom using a scale drawing of the room. Then, the groups will test the validity of their model by placing weathervanes that they have constructed around the room and opening a window or door. By observing the weather vanes, students will see how air actually flows through the room from a specific window or door. Students will then correct their model based on their experimental evidence. The following material list is given per group:
- One room with at least one window or door that can be opened (An optimal configuration would be one window or door per group.)
- Several pieces of construction paper (at least four per group)
- Strips of single ply tissue paper
- One tape measure (long enough to measure the dimensions of the room)
- Group size can vary depending on the number of windows/doors available and the number of students in the class.
- The room dimensions could be provided by the teacher. Also, students may need a brief introduction in how to make a drawing to scale.
- This is another opportunity to discuss controlled experiments in terms of why the students should hold the strips of tissue paper at the same height and in the same way. One student could also serve as a control and stand far away from the window/door or in another area that will not receive air flow from the window/door.
- You will probably need to coordinate this when multiple windows or doors are used. Only one window or door should be opened at a time for best results. Between openings, allow a short period (5 minutes) when all windows and doors are closed, if possible.
Answers to the Grasp Check will vary, but the air flow in the new window or door should be based on what the students observed in their experiment.
Scientific Laws and Theories
A scientific law is a description of a pattern in nature that is true in all circumstances that have been studied. That is, physical laws are meant to be universal , meaning that they apply throughout the known universe. Laws are often also concise, whereas theories are more complicated. A law can be expressed in the form of a single sentence or mathematical equation. For example, Newton’s second law of motion , which relates the motion of an object to the force applied ( F ), the mass of the object ( m ), and the object’s acceleration ( a ), is simply stated using the equation
Scientific ideas and explanations that are true in many, but not all situations in the universe are usually called principles . An example is Pascal’s principle , which explains properties of liquids, but not solids or gases. However, the distinction between laws and principles is sometimes not carefully made in science.
A theory is an explanation for patterns in nature that is supported by much scientific evidence and verified multiple times by multiple researchers. While many people confuse theories with educated guesses or hypotheses, theories have withstood more rigorous testing and verification than hypotheses.
[OL] Explain to students that in informal, everyday English the word theory can be used to describe an idea that is possibly true but that has not been proven to be true. This use of the word theory often leads people to think that scientific theories are nothing more than educated guesses. This is not just a misconception among students, but among the general public as well.
As a closing idea about scientific processes, we want to point out that scientific laws and theories, even those that have been supported by experiments for centuries, can still be changed by new discoveries. This is especially true when new technologies emerge that allow us to observe things that were formerly unobservable. Imagine how viewing previously invisible objects with a microscope or viewing Earth for the first time from space may have instantly changed our scientific theories and laws! What discoveries still await us in the future? The constant retesting and perfecting of our scientific laws and theories allows our knowledge of nature to progress. For this reason, many scientists are reluctant to say that their studies prove anything. By saying support instead of prove , it keeps the door open for future discoveries, even if they won’t occur for centuries or even millennia.
[OL] With regard to scientists avoiding using the word prove , the general public knows that science has proven certain things such as that the heart pumps blood and the Earth is round. However, scientists should shy away from using prove because it is impossible to test every single instance and every set of conditions in a system to absolutely prove anything. Using support or similar terminology leaves the door open for further discovery.
1 Atoms in Motion 1

| Science: |
| We are not concerned with where a new idea comes from – the sole test of its validity is experiment. |
| Atoms: |
| Things are made of myriads of particles about $10^{-8} $ cm in diameter. |
| They are in perpetual motion, the faster the hotter. |
| They stick together in complicated patterns. |
| They resist being pushed too close together. |
1–1 Introduction
This two-year course in physics is presented from the point of view that you, the reader, are going to be a physicist. This is not necessarily the case of course, but that is what every professor in every subject assumes! If you are going to be a physicist, you will have a lot to study: two hundred years of the most rapidly developing field of knowledge that there is. So much knowledge, in fact, that you might think that you cannot learn all of it in four years, and truly you cannot; you will have to go to graduate school too!
Surprisingly enough, in spite of the tremendous amount of work that has been done for all this time it is possible to condense the enormous mass of results to a large extent—that is, to find laws which summarize all our knowledge. Even so, the laws are so hard to grasp that it is unfair to you to start exploring this tremendous subject without some kind of map or outline of the relationship of one part of the subject of science to another. Following these preliminary remarks, the first three chapters will therefore outline the relation of physics to the rest of the sciences, the relations of the sciences to each other, and the meaning of science, to help us develop a “feel” for the subject.
You might ask why we cannot teach physics by just giving the basic laws on page one and then showing how they work in all possible circumstances, as we do in Euclidean geometry, where we state the axioms and then make all sorts of deductions. (So, not satisfied to learn physics in four years, you want to learn it in four minutes?) We cannot do it in this way for two reasons. First, we do not yet know all the basic laws: there is an expanding frontier of ignorance. Second, the correct statement of the laws of physics involves some very unfamiliar ideas which require advanced mathematics for their description. Therefore, one needs a considerable amount of preparatory training even to learn what the words mean. No, it is not possible to do it that way. We can only do it piece by piece.
Each piece, or part, of the whole of nature is always merely an approximation to the complete truth, or the complete truth so far as we know it. In fact, everything we know is only some kind of approximation, because we know that we do not know all the laws as yet. Therefore, things must be learned only to be unlearned again or, more likely, to be corrected.
The principle of science, the definition, almost, is the following: The test of all knowledge is experiment . Experiment is the sole judge of scientific “truth.” But what is the source of knowledge? Where do the laws that are to be tested come from? Experiment, itself, helps to produce these laws, in the sense that it gives us hints. But also needed is imagination to create from these hints the great generalizations—to guess at the wonderful, simple, but very strange patterns beneath them all, and then to experiment to check again whether we have made the right guess. This imagining process is so difficult that there is a division of labor in physics: there are theoretical physicists who imagine, deduce, and guess at new laws, but do not experiment; and then there are experimental physicists who experiment, imagine, deduce, and guess.
We said that the laws of nature are approximate: that we first find the “wrong” ones, and then we find the “right” ones. Now, how can an experiment be “wrong”? First, in a trivial way: if something is wrong with the apparatus that you did not notice. But these things are easily fixed, and checked back and forth. So without snatching at such minor things, how can the results of an experiment be wrong? Only by being inaccurate. For example, the mass of an object never seems to change: a spinning top has the same weight as a still one. So a “law” was invented: mass is constant, independent of speed. That “law” is now found to be incorrect. Mass is found to increase with velocity, but appreciable increases require velocities near that of light. A true law is: if an object moves with a speed of less than one hundred miles a second the mass is constant to within one part in a million. In some such approximate form this is a correct law. So in practice one might think that the new law makes no significant difference. Well, yes and no. For ordinary speeds we can certainly forget it and use the simple constant-mass law as a good approximation. But for high speeds we are wrong, and the higher the speed, the more wrong we are.
Finally, and most interesting, philosophically we are completely wrong with the approximate law. Our entire picture of the world has to be altered even though the mass changes only by a little bit. This is a very peculiar thing about the philosophy, or the ideas, behind the laws. Even a very small effect sometimes requires profound changes in our ideas.
Now, what should we teach first? Should we teach the correct but unfamiliar law with its strange and difficult conceptual ideas, for example the theory of relativity, four-dimensional space-time, and so on? Or should we first teach the simple “constant-mass” law, which is only approximate, but does not involve such difficult ideas? The first is more exciting, more wonderful, and more fun, but the second is easier to get at first, and is a first step to a real understanding of the first idea. This point arises again and again in teaching physics. At different times we shall have to resolve it in different ways, but at each stage it is worth learning what is now known, how accurate it is, how it fits into everything else, and how it may be changed when we learn more.
Let us now proceed with our outline, or general map, of our understanding of science today (in particular, physics, but also of other sciences on the periphery), so that when we later concentrate on some particular point we will have some idea of the background, why that particular point is interesting, and how it fits into the big structure. So, what is our overall picture of the world?
1–2 Matter is made of atoms
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generations of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis (or the atomic fact , or whatever you wish to call it) that all things are made of atoms—little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another . In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.
To illustrate the power of the atomic idea, suppose that we have a drop of water a quarter of an inch on the side. If we look at it very closely we see nothing but water—smooth, continuous water. Even if we magnify it with the best optical microscope available—roughly two thousand times—then the water drop will be roughly forty feet across, about as big as a large room, and if we looked rather closely, we would still see relatively smooth water—but here and there small football-shaped things swimming back and forth. Very interesting. These are paramecia. You may stop at this point and get so curious about the paramecia with their wiggling cilia and twisting bodies that you go no further, except perhaps to magnify the paramecia still more and see inside. This, of course, is a subject for biology, but for the present we pass on and look still more closely at the water material itself, magnifying it two thousand times again. Now the drop of water extends about fifteen miles across, and if we look very closely at it we see a kind of teeming, something which no longer has a smooth appearance—it looks something like a crowd at a football game as seen from a very great distance. In order to see what this teeming is about, we will magnify it another two hundred and fifty times and we will see something similar to what is shown in Fig. 1–1 . This is a picture of water magnified a billion times, but idealized in several ways. In the first place, the particles are drawn in a simple manner with sharp edges, which is inaccurate. Secondly, for simplicity, they are sketched almost schematically in a two-dimensional arrangement, but of course they are moving around in three dimensions. Notice that there are two kinds of “blobs” or circles to represent the atoms of oxygen (black) and hydrogen (white), and that each oxygen has two hydrogens tied to it. (Each little group of an oxygen with its two hydrogens is called a molecule.) The picture is idealized further in that the real particles in nature are continually jiggling and bouncing, turning and twisting around one another. You will have to imagine this as a dynamic rather than a static picture. Another thing that cannot be illustrated in a drawing is the fact that the particles are “stuck together”—that they attract each other, this one pulled by that one, etc. The whole group is “glued together,” so to speak. On the other hand, the particles do not squeeze through each other. If you try to squeeze two of them too close together, they repel.
The atoms are $1$ or $2\times10^{-8}$ cm in radius. Now $10^{-8}$ cm is called an angstrom (just as another name), so we say they are 1 or 2 angstroms (Å) in radius. Another way to remember their size is this: if an apple is magnified to the size of the earth, then the atoms in the apple are approximately the size of the original apple.
Now imagine this great drop of water with all of these jiggling particles stuck together and tagging along with each other. The water keeps its volume; it does not fall apart, because of the attraction of the molecules for each other. If the drop is on a slope, where it can move from one place to another, the water will flow, but it does not just disappear—things do not just fly apart—because of the molecular attraction. Now the jiggling motion is what we represent as heat : when we increase the temperature, we increase the motion. If we heat the water, the jiggling increases and the volume between the atoms increases, and if the heating continues there comes a time when the pull between the molecules is not enough to hold them together and they do fly apart and become separated from one another. Of course, this is how we manufacture steam out of water—by increasing the temperature; the particles fly apart because of the increased motion.
In Fig. 1–2 we have a picture of steam. This picture of steam fails in one respect: at ordinary atmospheric pressure there certainly would not be as many as three water molecules in this figure. Most squares this size would contain none—but we accidentally have two and a half or three in the picture (just so it would not be completely blank). Now in the case of steam we see the characteristic molecules more clearly than in the case of water. For simplicity, the molecules are drawn so that there is a $120^\circ$ angle between the hydrogen atoms. In actual fact the angle is $105^\circ3'$, and the distance between the center of a hydrogen and the center of the oxygen is 0.957 Å, so we know this molecule very well.
Let us see what some of the properties of steam vapor or any other gas are. The molecules, being separated from one another, will bounce against the walls. Imagine a room with a number of tennis balls (a hundred or so) bouncing around in perpetual motion. When they bombard the wall, this pushes the wall away. (Of course we would have to push the wall back.) This means that the gas exerts a jittery force which our coarse senses (not being ourselves magnified a billion times) feel only as an average push . In order to confine a gas we must apply a pressure. Figure 1–3 shows a standard vessel for holding gases (used in all textbooks), a cylinder with a piston in it. Now, it makes no difference what the shapes of water molecules are, so for simplicity we shall draw them as tennis balls or little dots. These things are in perpetual motion in all directions. So many of them are hitting the top piston all the time that to keep it from being patiently knocked out of the tank by this continuous banging, we shall have to hold the piston down by a certain force, which we call the pressure (really, the pressure times the area is the force). Clearly, the force is proportional to the area, for if we increase the area but keep the number of molecules per cubic centimeter the same, we increase the number of collisions with the piston in the same proportion as the area was increased.
Now let us put twice as many molecules in this tank, so as to double the density, and let them have the same speed, i.e., the same temperature. Then, to a close approximation, the number of collisions will be doubled, and since each will be just as “energetic” as before, the pressure is proportional to the density. If we consider the true nature of the forces between the atoms, we would expect a slight decrease in pressure because of the attraction between the atoms, and a slight increase because of the finite volume they occupy. Nevertheless, to an excellent approximation, if the density is low enough that there are not many atoms, the pressure is proportional to the density .
We can also see something else: If we increase the temperature without changing the density of the gas, i.e., if we increase the speed of the atoms, what is going to happen to the pressure? Well, the atoms hit harder because they are moving faster, and in addition they hit more often, so the pressure increases. You see how simple the ideas of atomic theory are.
Let us consider another situation. Suppose that the piston moves inward, so that the atoms are slowly compressed into a smaller space. What happens when an atom hits the moving piston? Evidently it picks up speed from the collision. You can try it by bouncing a ping-pong ball from a forward-moving paddle, for example, and you will find that it comes off with more speed than that with which it struck. (Special example: if an atom happens to be standing still and the piston hits it, it will certainly move.) So the atoms are “hotter” when they come away from the piston than they were before they struck it. Therefore all the atoms which are in the vessel will have picked up speed. This means that when we compress a gas slowly, the temperature of the gas increases . So, under slow compression , a gas will increase in temperature, and under slow expansion it will decrease in temperature.
We now return to our drop of water and look in another direction. Suppose that we decrease the temperature of our drop of water. Suppose that the jiggling of the molecules of the atoms in the water is steadily decreasing. We know that there are forces of attraction between the atoms, so that after a while they will not be able to jiggle so well. What will happen at very low temperatures is indicated in Fig. 1–4 : the molecules lock into a new pattern which is ice . This particular schematic diagram of ice is wrong because it is in two dimensions, but it is right qualitatively. The interesting point is that the material has a definite place for every atom , and you can easily appreciate that if somehow or other we were to hold all the atoms at one end of the drop in a certain arrangement, each atom in a certain place, then because of the structure of interconnections, which is rigid, the other end miles away (at our magnified scale) will have a definite location. So if we hold a needle of ice at one end, the other end resists our pushing it aside, unlike the case of water, in which the structure is broken down because of the increased jiggling so that the atoms all move around in different ways. The difference between solids and liquids is, then, that in a solid the atoms are arranged in some kind of an array, called a crystalline array , and they do not have a random position at long distances; the position of the atoms on one side of the crystal is determined by that of other atoms millions of atoms away on the other side of the crystal. Figure 1–4 is an invented arrangement for ice, and although it contains many of the correct features of ice, it is not the true arrangement. One of the correct features is that there is a part of the symmetry that is hexagonal. You can see that if we turn the picture around an axis by $60^\circ$, the picture returns to itself. So there is a symmetry in the ice which accounts for the six-sided appearance of snowflakes. Another thing we can see from Fig. 1–4 is why ice shrinks when it melts. The particular crystal pattern of ice shown here has many “holes” in it, as does the true ice structure. When the organization breaks down, these holes can be occupied by molecules. Most simple substances, with the exception of water and type metal, expand upon melting, because the atoms are closely packed in the solid crystal and upon melting need more room to jiggle around, but an open structure collapses, as in the case of water.
Now although ice has a “rigid” crystalline form, its temperature can change—ice has heat. If we wish, we can change the amount of heat. What is the heat in the case of ice? The atoms are not standing still. They are jiggling and vibrating. So even though there is a definite order to the crystal—a definite structure—all of the atoms are vibrating “in place.” As we increase the temperature, they vibrate with greater and greater amplitude, until they shake themselves out of place. We call this melting . As we decrease the temperature, the vibration decreases and decreases until, at absolute zero, there is a minimum amount of vibration that the atoms can have, but not zero . This minimum amount of motion that atoms can have is not enough to melt a substance, with one exception: helium. Helium merely decreases the atomic motions as much as it can, but even at absolute zero there is still enough motion to keep it from freezing. Helium, even at absolute zero, does not freeze, unless the pressure is made so great as to make the atoms squash together. If we increase the pressure, we can make it solidify.
1–3 Atomic processes
So much for the description of solids, liquids, and gases from the atomic point of view. However, the atomic hypothesis also describes processes , and so we shall now look at a number of processes from an atomic standpoint. The first process that we shall look at is associated with the surface of the water. What happens at the surface of the water? We shall now make the picture more complicated—and more realistic—by imagining that the surface is in air. Figure 1–5 shows the surface of water in air. We see the water molecules as before, forming a body of liquid water, but now we also see the surface of the water. Above the surface we find a number of things: First of all there are water molecules, as in steam. This is water vapor , which is always found above liquid water. (There is an equilibrium between the steam vapor and the water which will be described later.) In addition we find some other molecules—here two oxygen atoms stuck together by themselves, forming an oxygen molecule , there two nitrogen atoms also stuck together to make a nitrogen molecule. Air consists almost entirely of nitrogen, oxygen, some water vapor, and lesser amounts of carbon dioxide, argon, and other things. So above the water surface is the air, a gas, containing some water vapor. Now what is happening in this picture? The molecules in the water are always jiggling around. From time to time, one on the surface happens to be hit a little harder than usual, and gets knocked away. It is hard to see that happening in the picture because it is a still picture. But we can imagine that one molecule near the surface has just been hit and is flying out, or perhaps another one has been hit and is flying out. Thus, molecule by molecule, the water disappears—it evaporates. But if we close the vessel above, after a while we shall find a large number of molecules of water amongst the air molecules. From time to time, one of these vapor molecules comes flying down to the water and gets stuck again. So we see that what looks like a dead, uninteresting thing—a glass of water with a cover, that has been sitting there for perhaps twenty years—really contains a dynamic and interesting phenomenon which is going on all the time. To our eyes, our crude eyes, nothing is changing, but if we could see it a billion times magnified, we would see that from its own point of view it is always changing: molecules are leaving the surface, molecules are coming back.
Why do we see no change ? Because just as many molecules are leaving as are coming back! In the long run “nothing happens.” If we then take the top of the vessel off and blow the moist air away, replacing it with dry air, then the number of molecules leaving is just the same as it was before, because this depends on the jiggling of the water, but the number coming back is greatly reduced because there are so many fewer water molecules above the water. Therefore there are more going out than coming in, and the water evaporates. Hence, if you wish to evaporate water turn on the fan!
Here is something else: Which molecules leave? When a molecule leaves it is due to an accidental, extra accumulation of a little bit more than ordinary energy, which it needs if it is to break away from the attractions of its neighbors. Therefore, since those that leave have more energy than the average, the ones that are left have less average motion than they had before. So the liquid gradually cools if it evaporates. Of course, when a molecule of vapor comes from the air to the water below there is a sudden great attraction as the molecule approaches the surface. This speeds up the incoming molecule and results in generation of heat. So when they leave they take away heat; when they come back they generate heat. Of course when there is no net evaporation the result is nothing—the water is not changing temperature. If we blow on the water so as to maintain a continuous preponderance in the number evaporating, then the water is cooled. Hence, blow on soup to cool it!
Of course you should realize that the processes just described are more complicated than we have indicated. Not only does the water go into the air, but also, from time to time, one of the oxygen or nitrogen molecules will come in and “get lost” in the mass of water molecules, and work its way into the water. Thus the air dissolves in the water; oxygen and nitrogen molecules will work their way into the water and the water will contain air. If we suddenly take the air away from the vessel, then the air molecules will leave more rapidly than they come in, and in doing so will make bubbles. This is very bad for divers, as you may know.
Now we go on to another process. In Fig. 1–6 we see, from an atomic point of view, a solid dissolving in water. If we put a crystal of salt in the water, what will happen? Salt is a solid, a crystal, an organized arrangement of “salt atoms.” Figure 1–7 is an illustration of the three-dimensional structure of common salt, sodium chloride. Strictly speaking, the crystal is not made of atoms, but of what we call ions . An ion is an atom which either has a few extra electrons or has lost a few electrons. In a salt crystal we find chlorine ions (chlorine atoms with an extra electron) and sodium ions (sodium atoms with one electron missing). The ions all stick together by electrical attraction in the solid salt, but when we put them in the water we find, because of the attractions of the negative oxygen and positive hydrogen for the ions, that some of the ions jiggle loose. In Fig. 1–6 we see a chlorine ion getting loose, and other atoms floating in the water in the form of ions. This picture was made with some care. Notice, for example, that the hydrogen ends of the water molecules are more likely to be near the chlorine ion, while near the sodium ion we are more likely to find the oxygen end, because the sodium is positive and the oxygen end of the water is negative, and they attract electrically. Can we tell from this picture whether the salt is dissolving in water or crystallizing out of water? Of course we cannot tell, because while some of the atoms are leaving the crystal other atoms are rejoining it. The process is a dynamic one, just as in the case of evaporation, and it depends on whether there is more or less salt in the water than the amount needed for equilibrium. By equilibrium we mean that situation in which the rate at which atoms are leaving just matches the rate at which they are coming back. If there is almost no salt in the water, more atoms leave than return, and the salt dissolves. If, on the other hand, there are too many “salt atoms,” more return than leave, and the salt is crystallizing.
In passing, we mention that the concept of a molecule of a substance is only approximate and exists only for a certain class of substances. It is clear in the case of water that the three atoms are actually stuck together. It is not so clear in the case of sodium chloride in the solid. There is just an arrangement of sodium and chlorine ions in a cubic pattern. There is no natural way to group them as “molecules of salt.”
Returning to our discussion of solution and precipitation, if we increase the temperature of the salt solution, then the rate at which atoms are taken away is increased, and so is the rate at which atoms are brought back. It turns out to be very difficult, in general, to predict which way it is going to go, whether more or less of the solid will dissolve. Most substances dissolve more, but some substances dissolve less, as the temperature increases.
1–4 Chemical reactions
In all of the processes which have been described so far, the atoms and the ions have not changed partners, but of course there are circumstances in which the atoms do change combinations, forming new molecules. This is illustrated in Fig. 1–8 . A process in which the rearrangement of the atomic partners occurs is what we call a chemical reaction . The other processes so far described are called physical processes, but there is no sharp distinction between the two. (Nature does not care what we call it, she just keeps on doing it.) This figure is supposed to represent carbon burning in oxygen. In the case of oxygen, two oxygen atoms stick together very strongly. (Why do not three or even four stick together? That is one of the very peculiar characteristics of such atomic processes. Atoms are very special: they like certain particular partners, certain particular directions, and so on. It is the job of physics to analyze why each one wants what it wants. At any rate, two oxygen atoms form, saturated and happy, a molecule.)
The carbon atoms are supposed to be in a solid crystal (which could be graphite or diamond 2 ). Now, for example, one of the oxygen molecules can come over to the carbon, and each atom can pick up a carbon atom and go flying off in a new combination—“carbon-oxygen”—which is a molecule of the gas called carbon monoxide. It is given the chemical name CO. It is very simple: the letters “CO” are practically a picture of that molecule. But carbon attracts oxygen much more than oxygen attracts oxygen or carbon attracts carbon. Therefore in this process the oxygen may arrive with only a little energy, but the oxygen and carbon will snap together with a tremendous vengeance and commotion, and everything near them will pick up the energy. A large amount of motion energy, kinetic energy, is thus generated. This of course is burning ; we are getting heat from the combination of oxygen and carbon. The heat is ordinarily in the form of the molecular motion of the hot gas, but in certain circumstances it can be so enormous that it generates light . That is how one gets flames .
In addition, the carbon monoxide is not quite satisfied. It is possible for it to attach another oxygen, so that we might have a much more complicated reaction in which the oxygen is combining with the carbon, while at the same time there happens to be a collision with a carbon monoxide molecule. One oxygen atom could attach itself to the CO and ultimately form a molecule, composed of one carbon and two oxygens, which is designated CO$_2$ and called carbon dioxide. If we burn the carbon with very little oxygen in a very rapid reaction (for example, in an automobile engine, where the explosion is so fast that there is not time for it to make carbon dioxide) a considerable amount of carbon monoxide is formed. In many such rearrangements, a very large amount of energy is released, forming explosions, flames, etc., depending on the reactions. Chemists have studied these arrangements of the atoms, and found that every substance is some type of arrangement of atoms .
To illustrate this idea, let us consider another example. If we go into a field of small violets, we know what “that smell” is. It is some kind of molecule , or arrangement of atoms, that has worked its way into our noses. First of all, how did it work its way in? That is rather easy. If the smell is some kind of molecule in the air, jiggling around and being knocked every which way, it might have accidentally worked its way into the nose. Certainly it has no particular desire to get into our nose. It is merely one helpless part of a jostling crowd of molecules, and in its aimless wanderings this particular chunk of matter happens to find itself in the nose.
Now chemists can take special molecules like the odor of violets, and analyze them and tell us the exact arrangement of the atoms in space. We know that the carbon dioxide molecule is straight and symmetrical: O—C—O. (That can be determined easily, too, by physical methods.) However, even for the vastly more complicated arrangements of atoms that there are in chemistry, one can, by a long, remarkable process of detective work, find the arrangements of the atoms. Figure 1–9 is a picture of the air in the neighborhood of a violet; again we find nitrogen and oxygen in the air, and water vapor. (Why is there water vapor? Because the violet is wet . All plants transpire.) However, we also see a “monster” composed of carbon atoms, hydrogen atoms, and oxygen atoms, which have picked a certain particular pattern in which to be arranged. It is a much more complicated arrangement than that of carbon dioxide; in fact, it is an enormously complicated arrangement. Unfortunately, we cannot picture all that is really known about it chemically, because the precise arrangement of all the atoms is actually known in three dimensions, while our picture is in only two dimensions. The six carbons which form a ring do not form a flat ring, but a kind of “puckered” ring. All of the angles and distances are known. So a chemical formula is merely a picture of such a molecule. When the chemist writes such a thing on the blackboard, he is trying to “draw,” roughly speaking, in two dimensions. For example, we see a “ring” of six carbons, and a “chain” of carbons hanging on the end, with an oxygen second from the end, three hydrogens tied to that carbon, two carbons and three hydrogens sticking up here, etc.
How does the chemist find what the arrangement is? He mixes bottles full of stuff together, and if it turns red, it tells him that it consists of one hydrogen and two carbons tied on here; if it turns blue, on the other hand, that is not the way it is at all. This is one of the most fantastic pieces of detective work that has ever been done—organic chemistry. To discover the arrangement of the atoms in these enormously complicated arrays the chemist looks at what happens when he mixes two different substances together. The physicist could never quite believe that the chemist knew what he was talking about when he described the arrangement of the atoms. For about twenty years it has been possible, in some cases, to look at such molecules (not quite as complicated as this one, but some which contain parts of it) by a physical method, and it has been possible to locate every atom, not by looking at colors, but by measuring where they are . And lo and behold!, the chemists are almost always correct.
It turns out, in fact, that in the odor of violets there are three slightly different molecules, which differ only in the arrangement of the hydrogen atoms.
One problem of chemistry is to name a substance, so that we will know what it is. Find a name for this shape! Not only must the name tell the shape, but it must also tell that here is an oxygen atom, there a hydrogen—exactly what and where each atom is. So we can appreciate that the chemical names must be complex in order to be complete. You see that the name of this thing in the more complete form that will tell you the structure of it is 4-(2, 2, 3, 6 tetramethyl-5-cyclohexenyl)-3-buten-2-one, and that tells you that this is the arrangement. We can appreciate the difficulties that the chemists have, and also appreciate the reason for such long names. It is not that they wish to be obscure, but they have an extremely difficult problem in trying to describe the molecules in words!
How do we know that there are atoms? By one of the tricks mentioned earlier: we make the hypothesis that there are atoms, and one after the other results come out the way we predict, as they ought to if things are made of atoms. There is also somewhat more direct evidence, a good example of which is the following: The atoms are so small that you cannot see them with a light microscope—in fact, not even with an electron microscope. (With a light microscope you can only see things which are much bigger.) Now if the atoms are always in motion, say in water, and we put a big ball of something in the water, a ball much bigger than the atoms, the ball will jiggle around—much as in a push ball game, where a great big ball is pushed around by a lot of people. The people are pushing in various directions, and the ball moves around the field in an irregular fashion. So, in the same way, the “large ball” will move because of the inequalities of the collisions on one side to the other, from one moment to the next. Therefore, if we look at very tiny particles (colloids) in water through an excellent microscope, we see a perpetual jiggling of the particles, which is the result of the bombardment of the atoms. This is called the Brownian motion .
We can see further evidence for atoms in the structure of crystals. In many cases the structures deduced by x-ray analysis agree in their spatial “shapes” with the forms actually exhibited by crystals as they occur in nature. The angles between the various “faces” of a crystal agree, within seconds of arc, with angles deduced on the assumption that a crystal is made of many “layers” of atoms.
Everything is made of atoms . That is the key hypothesis. The most important hypothesis in all of biology, for example, is that everything that animals do, atoms do . In other words, there is nothing that living things do that cannot be understood from the point of view that they are made of atoms acting according to the laws of physics . This was not known from the beginning: it took some experimenting and theorizing to suggest this hypothesis, but now it is accepted, and it is the most useful theory for producing new ideas in the field of biology.
If a piece of steel or a piece of salt, consisting of atoms one next to the other, can have such interesting properties; if water—which is nothing but these little blobs, mile upon mile of the same thing over the earth—can form waves and foam, and make rushing noises and strange patterns as it runs over cement; if all of this, all the life of a stream of water, can be nothing but a pile of atoms, how much more is possible ? If instead of arranging the atoms in some definite pattern, again and again repeated, on and on, or even forming little lumps of complexity like the odor of violets, we make an arrangement which is always different from place to place, with different kinds of atoms arranged in many ways, continually changing, not repeating, how much more marvelously is it possible that this thing might behave? Is it possible that that “thing” walking back and forth in front of you, talking to you, is a great glob of these atoms in a very complex arrangement, such that the sheer complexity of it staggers the imagination as to what it can do? When we say we are a pile of atoms, we do not mean we are merely a pile of atoms, because a pile of atoms which is not repeated from one to the other might well have the possibilities which you see before you in the mirror.
- The original tape recording of this lecture suffered some damage in the making, so it sounds 'clipped' in many places, particularly when Feynman speaks loudly. (This is the only recording in the collection that is damaged.) An illustrated transcript of the lecture can be found here . ↩
- One can burn a diamond in air. ↩
By Guest Blogger
The Most Important Sentence
December 2, 2015

In his Lectures on Physics , Richard Feynman imagines the following scenario: some cataclysm destroys all scientific knowledge, but modern scientists have the opportunity to pass down a single sentence to future generations. What should that sentence be? What single statement would allow future generations to rebuild science?
That sentence, Feynman argues, would state the atomic hypothesis, “that all things are made of atoms – little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another.” If we had only the atomic hypothesis, he suggests, "just a little imagination and thinking" would tell us an enormous amount about the world.
Is Feynman right? Could we reconstitute all of modern science on the basis of the atomic hypothesis? A brief look at the history of atomism suggests that he might have been on to something.
The atomic hypothesis isn’t new – it was first proposed in the West in the 5th century BCE by the Greek philosopher Democritus . Using just the idea that matter can be broken up into tiny indivisible parts, Democritus and other ancient atomists figured out an astonishing amount about the world. Although most of their writings are lost, we have one surviving account of the ancient atomists’ theories in a six-book Latin poem called De Rerum Natura , written by the Roman poet Lucretius . Lucretius, like the other atomists, applied “just a little imagination and thinking” (to quote Feynman) to the atomic hypothesis in an attempt to explain the natural world. Though he gets some facts wrong, it’s amazing how much he gets right: he proposes that the earth occupies an insignificant point in a potentially infinite universe, that life evolved through natural selection, that if life evolved on earth there’s no reason to think it didn’t evolve on other planets, and that the human species doesn’t occupy a unique point in time and will eventually evolve into other organisms. These are some remarkably prescient ideas.
Unfortunately, the Hellenistic world faced just the sort of cataclysm that Feynman imagines: the Roman Empire fell, most classical knowledge was destroyed or forgotten, and intellectual activity stagnated for millennia. But, as fate would have it, the atomic hypothesis just barely survived the catastrophe. One and a half thousand years after the time of Lucretius, an Italian humanist named Poggio Bracciolini discovered what was likely the last surviving copy of De Rerum Natura in a remote German monastery. Recognizing that the poem was a window into Classical thought, Poggio copied the poem and sent it to a friend, who himself made a copy, and so on. Thus, very slowly, the poem began to circulate again in Europe. It took centuries before the ideas found in De Rerum Natura could be discussed openly, since to Christian Europe Lucretius’ arguments were seen as heretical, but the damage was done: the atomic hypothesis reentered the stream of Western thought.
Harvard professor Stephen Greenblatt has famously argued that the rediscovery of De Rerum Natura paved the way to modernity, since Renaissance and Enlightenment-era thinkers used the idea of atomism to construct much of modern science and philosophy. If Greenblatt is right, then history validates Feynman’s argument – historically, the atomic hypothesis did help us reconstitute science.
But there are two substantial problems with this picture. The first is that science wasn’t actually revived because of the rediscovery of the atomic hypothesis, but was instead resurrected in the early Renaissance because of empirical findings that went against received wisdom: Copernicus came up with a mathematically elegant heliocentric model that went against Ptolemaic geocentrism, Galileo discovered sunspots that went against Aristotle’s idea that the heavens were perfect, and the discovery of new continents proved that Ptolemy was wrong about the number of continents. These and other findings inspired thinkers in the late Renaissance and Enlightenment to jettison the ideas of the Greeks and Romans. They realized that if they wanted to understand how the world works, they had to figure it out for themselves. A letter from Isaac Newton to the secretary of the Royal Society captures the spirit of the time: “The best and safest method of philosophizing seems to be, first, to inquire diligently into the properties of things and to establish those properties by experiments, and to proceed later to hypotheses for the explanation of things themselves.”
It was this emphasis on empirical testing that drove the Scientific Revolution, not the atomic hypothesis. Sure, assuming that things can be broken down into smaller things can tell us a lot about the world – so much so is attested not only by the remarkable prescience of the ideas outlined in De Rerum Natura , but also by the equally prescient ideas of the Ancient Indian atomists . But, ultimately, the ideas of the Greek, Roman, and Indian atomists were just philosophical – the ancients almost never bothered to actually test their theories. So while the atomic hypothesis proved remarkably fruitful for Renaissance and Enlightenment-era scientists, the only reason their ideas are considered scientific rather than philosophical is because they verified their theories through experimentation.
The second problem with Feynman’s argument is that, strictly speaking, the atomic hypothesis turns out to be false. Quantum field theory – a discipline that Feynman had a key role in developing – has thrown the whole edifice of atomism out the window . Subatomic particles aren’t actually particles, but instead are just local excitations of quantum fields. While fields are still in a vague sense “physical,” since they have properties like momentum and energy, they’re certainly not atoms. In light of contemporary physics, a more accurate description of the ultimate composition of things isn’t to say that everything is made up of atoms, but rather to say that, more generally, everything can be decomposed into the behavior of other things: a person isn’t a self-same thing but rather the behavior of cells, a cell isn’t a self-same thing but rather the behavior of molecules, a molecule isn’t a self-same thing but rather the behavior of atoms, an atom isn’t a self-same thing but rather the behavior of subatomic particles, a subatomic particle isn’t a self-same thing but rather the behavior of quantum fields, etc. Who knows where, or even if, it bottoms out.
So if there were some cataclysm today that destroyed all scientific knowledge and we had the opportunity to pass on just a single sentence to the next generation, I think it should be this: “Every object can be decomposed into the behavior of other objects, and to be certain that your understanding of how objects behave is correct, you have to form hypotheses about that behavior and test those hypotheses through experimentation.” If future generations had just that one sentence, a little thinking and imagination could reveal to them a whole lot about the world.
Feature image courtesy Pelf, public domain .
Related Articles
Staff listing, fall 2017.
By The Berkeley Science Review
From the Editor
By Emily Hartman
From the Field
By Karina Klonoski

Most Popular
Astrocytes: the defenders of the you-niverse.
By Deepthi Bannai
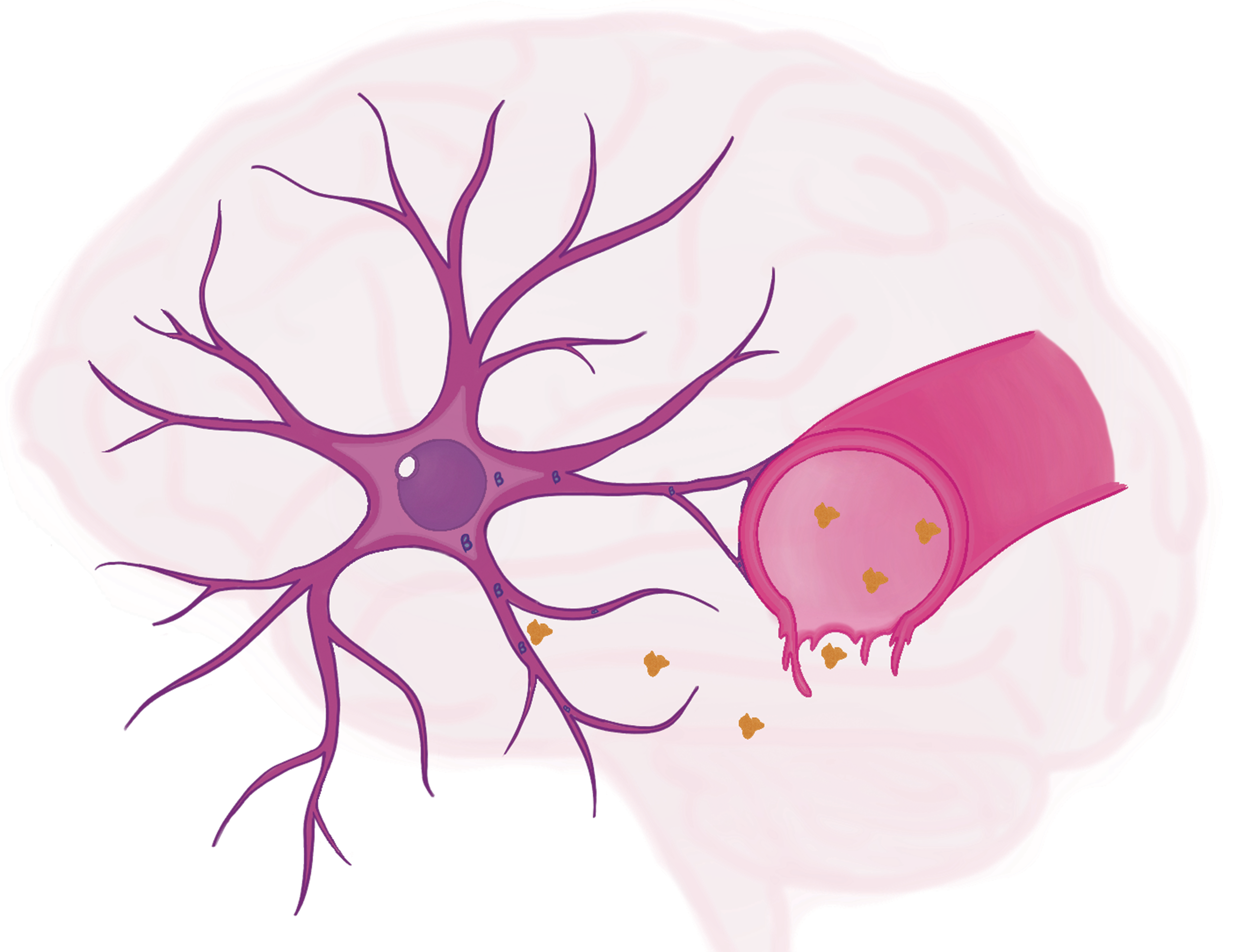
From the Field - A tale of two chipmunks
By Kwasi Wrensford
Notice something wrong?
Please report it here.
Richard Feynman on the One Sentence to Be Passed on to the Next Generation
By maria popova.

From the very beginning of his first-ever lecture comes this timeless gem (mentioned in Daniel Bor’s excellent The Ravenous Brain ) that set the tone for both Feynman’s academic contribution and his broader cultural legacy:
If, in some cataclysm, all of scientific knowledge were to be destroyed, and only one sentence passed on to the next generation of creatures, what statement would contain the most information in the fewest words? I believe it is the atomic hypothesis that all things are made of atoms — little particles that move around in perpetual motion, attracting each other when they are a little distance apart, but repelling upon being squeezed into one another. In that one sentence, you will see, there is an enormous amount of information about the world, if just a little imagination and thinking are applied.
Couple with Feynman’s ode to the wonder of life, performed by Yo-Yo Ma in a breathtaking animation , and his famed ode to a flower , then revisit Bertrand Russell on science as the key to democracy .
— Published September 11, 2012 — https://www.themarginalian.org/2012/09/11/richard-feynman-lectures-on-physics/ —

www.themarginalian.org

PRINT ARTICLE
Email article, filed under, culture education richard feynman science, view full site.
The Marginalian participates in the Bookshop.org and Amazon.com affiliate programs, designed to provide a means for sites to earn commissions by linking to books. In more human terms, this means that whenever you buy a book from a link here, I receive a small percentage of its price, which goes straight back into my own colossal biblioexpenses. Privacy policy . (TLDR: You're safe — there are no nefarious "third parties" lurking on my watch or shedding crumbs of the "cookies" the rest of the internet uses.)
How to Use hypothesis in a Sentence
- The results of the experiment did not support his hypothesis .
- Their hypothesis is that watching excessive amounts of television reduces a person's ability to concentrate.
Some of these examples are programmatically compiled from various online sources to illustrate current usage of the word 'hypothesis.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!

IMAGES
VIDEO
COMMENTS
Step 3. Formulate your hypothesis. Now you should have some idea of what you expect to find. Write your initial answer to the question in a clear, concise sentence. Example: Formulating your hypothesis Attending more lectures leads to better exam results. Tip AI tools like ChatGPT can be effectively used to brainstorm potential hypotheses.
Here are some research hypothesis examples: If you leave the lights on, then it takes longer for people to fall asleep. If you refrigerate apples, they last longer before going bad. If you keep the curtains closed, then you need less electricity to heat or cool the house (the electric bill is lower). If you leave a bucket of water uncovered ...
A "hypothesis" is a consequence of the theory that one can test. From Chloë's Theory, we have the hypothesis that an object will take 2-√ 2 times longer to fall from 1m 1 m than from 2 m 2 m. We can formulate the hypothesis based on the theory and then test that hypothesis. If the hypothesis is found to be invalidated by experiment ...
Good Hypothesis : Poor Hypothesis: When there is less oxygen in the water, rainbow trout suffer more lice. Kristin says: "This hypothesis is good because it is testable, simple, written as a statement, and establishes the participants (trout), variables (oxygen in water, and numbers of lice), and predicts effect (as oxygen levels go down, the numbers of lice go up)."
Here are examples of a scientific hypothesis and how to improve a hypothesis to use it for an experiment. ... B.A., Physics and Mathematics, Hastings College; Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels.
hypothesis. science. scientific hypothesis, an idea that proposes a tentative explanation about a phenomenon or a narrow set of phenomena observed in the natural world. The two primary features of a scientific hypothesis are falsifiability and testability, which are reflected in an "If…then" statement summarizing the idea and in the ...
Learning how to write a hypothesis comes down to knowledge and strategy. So where do you start? Learn how to make your hypothesis strong step-by-step here.
A hypothesis (plural hypotheses) is a proposed explanation for an observation. The definition depends on the subject. In science, a hypothesis is part of the scientific method. It is a prediction or explanation that is tested by an experiment. Observations and experiments may disprove a scientific hypothesis, but can never entirely prove one.
Step 3: Formulate your hypothesis. Now you should have some idea of what you expect to find. Write your initial answer to the question in a clear, concise sentence. Example: Initial expectations Attending more lectures leads to better exam results. Step 4: Refine your hypothesis. You need to make sure your hypothesis is specific and testable.
Bibliography. A scientific hypothesis is a tentative, testable explanation for a phenomenon in the natural world. It's the initial building block in the scientific method. Many describe it as an ...
Functions of Hypothesis. Following are the functions performed by the hypothesis: Hypothesis helps in making an observation and experiments possible. It becomes the start point for the investigation. Hypothesis helps in verifying the observations. It helps in directing the inquiries in the right direction.
Define a scientific model and describe examples of physical and mathematical models used in physics; Compare and contrast hypothesis, theory, and law; Teacher Support. ... A law can be expressed in the form of a single sentence or mathematical equation. For example, Newton's second law of motion, which relates the motion of an object to ...
A hypothesis is often called an "educated guess," but this is an oversimplification. An example of a hypothesis would be: "If snake species A and B compete for the same resources, and if we ...
The hypothesis of Andreas Cellarius, showing the planetary motions in eccentric and epicyclical orbits. A hypothesis (pl.: hypotheses) is a proposed explanation for a phenomenon.For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous observations that cannot satisfactorily be explained with ...
A hypothesis is a tentative, testable answer to a scientific question. Once a scientist has a scientific question she is interested in, the scientist reads up to find out what is already known on the topic. Then she uses that information to form a tentative answer to her scientific question. Sometimes people refer to the tentative answer as "an ...
To form a solid theory, the vital first step is creating a hypothesis. See the various types of hypotheses and how they can lead you on the path to discovery. To form a solid theory, the vital first step is creating a hypothesis. ... Sentences Grammar Vocabulary Usage Reading & Writing ...
That is the key hypothesis. The most important hypothesis in all of biology, for example, is that everything that animals do, atoms do. In other words, there is nothing that living things do that cannot be understood from the point of view that they are made of atoms acting according to the laws of physics. This was not known from the beginning ...
The meaning of HYPOTHESIS is an assumption or concession made for the sake of argument. How to use hypothesis in a sentence. The Difference Between Hypothesis and Theory Synonym Discussion of Hypothesis. ... "Physics and Chemistry of Comets," in Encyclopedia of the Solar System Paul R. Weissman et al., editors, 1999. As ...
In his Lectures on Physics, Richard Feynman imagines the following scenario: some cataclysm destroys all scientific knowledge, but modern scientists have the opportunity to pass down a single sentence to future generations.What should that sentence be? What single statement would allow future generations to rebuild science? That sentence, Feynman argues, would state the atomic hypothesis ...
The great Richard Feynman (May 11, 1918-February 15, 1988) — champion of scientific culture, graphic novel hero, crusader for integrity, holder of the key to science — may have earned himself the moniker "the Great Explainer," but when Caltech invited him to take over the introductory course in physics in 1961, they took an enormous chance on a theoretical physicist with no ...
Their hypothesis is that watching excessive amounts of television reduces a person's ability to concentrate. The coming days and weeks will put that hypothesis to the test. —. Jonathan Wosen, STAT , 14 Nov. 2022. And my hypothesis is that this has changed the way the midterm electorate thinks. —.